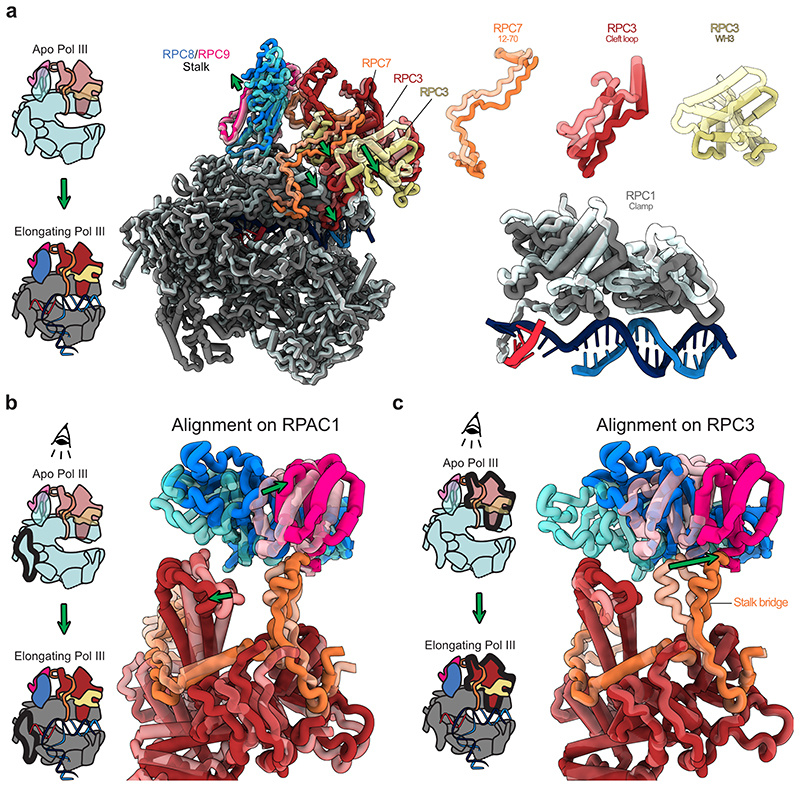
Extended Data Fig. 9. RPC7 tethers heterotrimer and stalk that move in opposite directions.
Protein elements are shown as thick ribbon to better visualize conformational changes. a, Left: Schematic showing the transition from DNA-unbound Apo Pol III to DNA/RNA-bound elongating Pol III. Middle: Side view showing the superimposition of apo and elongating human Pol III with RPAC1 being used for aligning the two structures. Apo Pol and elongating human Pol III are shown as transparent and solid ribbons, respectively. Green arrows illustrate movements of Pol III heterotrimer and clamp and the polymerase stalk domain. Right: Close-up views on selected moving elements showing that heterotrimer and clamp move towards the bound downstream DNA upon transition from apo to elongating Pol III. b, c, Top views onto the heterotrimer and stalk domains with two different subunits (RPAC1 in b and RPC3 in c) used to align apo and elongating human Pol III structures. Aligned subunits are outlined in bold in the associated schematics shown on the left. b, The RPC7 stalk bridge tethers heterotrimer and stalk although they the two domains move in opposite directions. c, The tip of the RPC7 stalk bridge is anchored to the stalk domain and moves in the same direction.