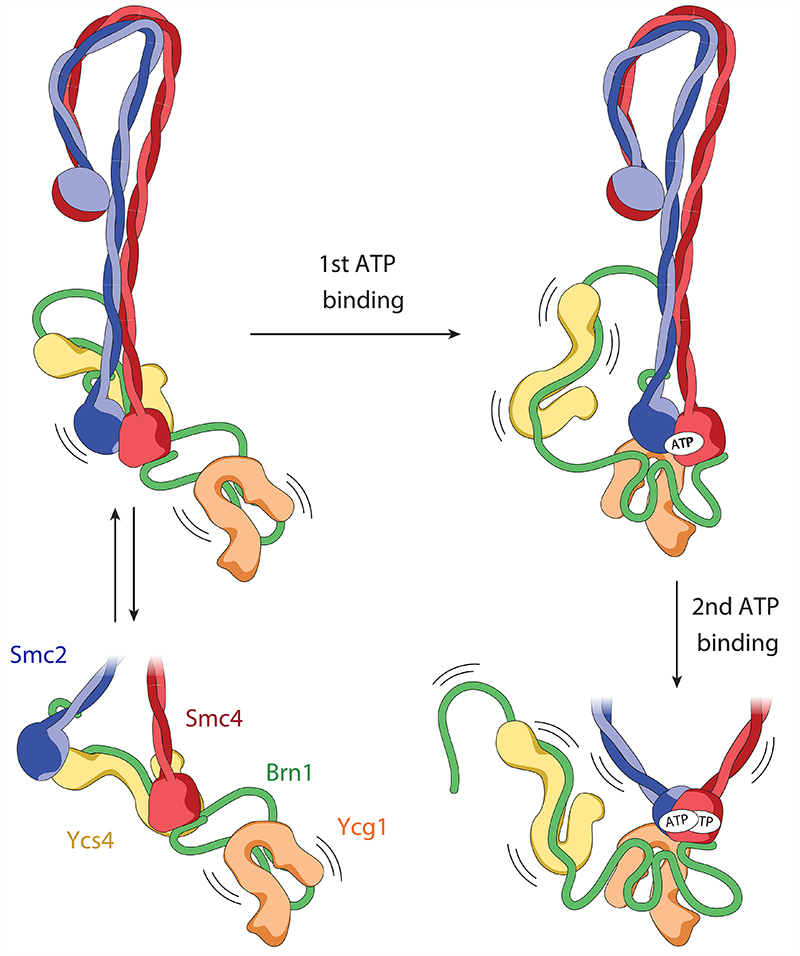
Fig. 6. Flip-flop model of the condensin reaction cycle.
In the absence of ATP, the Smc2 and Smc4 ATPase domains are either separated by ~2 nm, with Ycs4 bound to the Smc4 head (non-engaged state) or separated by ~10 nm with Ycs4 bound to both, the Smc2 and the Smc4 heads (bridged state). In both states, Ycg1 is flexibly attached to the rest of the complex only via its binding to the central region of Brn1. ATP binding to the Smc4 active site releases Ycs4, which enables Ycg1 binding to Smc2 and initiates Smc2–Smc4 head engagement. The head domains then fully engage upon ATP binding at the Smc2 active site. Kinking of the Smc2 coiled coil triggers release of Brn1N and (at least partially) opens the coiled coils from their rod-shaped conformation.