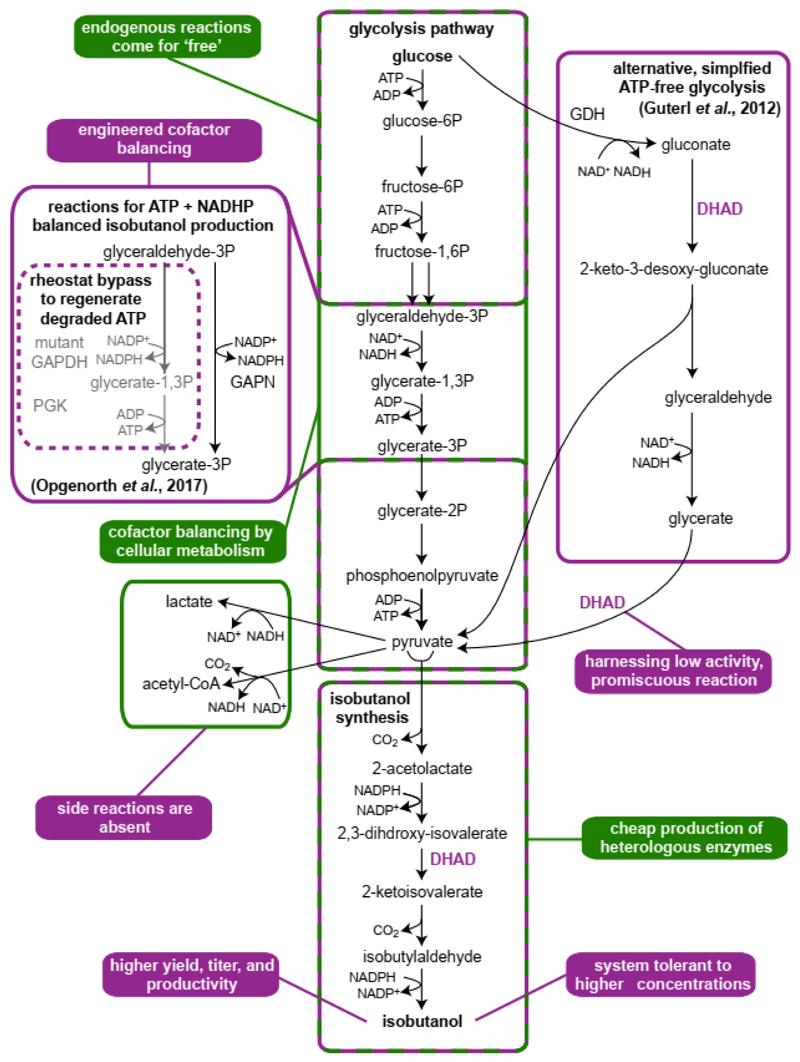
Figure 2. Isobutanol production exemplifies advantages of cellular and cell-free approaches.
Key advantages and pathways are color-marked green for cellular production and purple for cell-free production. Isobutanol production in engineered in vivo in E. coli reaches a titer of 23 g/L and 86% of the theoretical yield [78]. The first in vitro demonstration of isobutanol production was based on an ATP-independent glycolysis pathway, including the promiscuous gluconate and glycerate dehydrogenase activity of di-hydroxy-acid dehydratase (DHAD) [12]. A more recent in vitro system achieved dynamic cofactor balancing by a glycolytic ‘rheostat’ that combines a non-phosphorylating, NADPH-regenerating GapN with a mutant GapDH that can regenerate NADPH while generating ATP [31]. This design enabled dynamic balancing of cofactor consumption and regeneration during isobutanol production and supported a high isobutanol titer of 24 g/L at 91.5% theoretical yield. While isobutanol toxicity was a major limitation of cellular production, in vitro production could sustain higher titer with little adversary effects. However, the cell-free system is stable for 48 days due to enzyme inactivation, emphasizing the advantage of cellular production, in which the catalysts are produced at low cost.