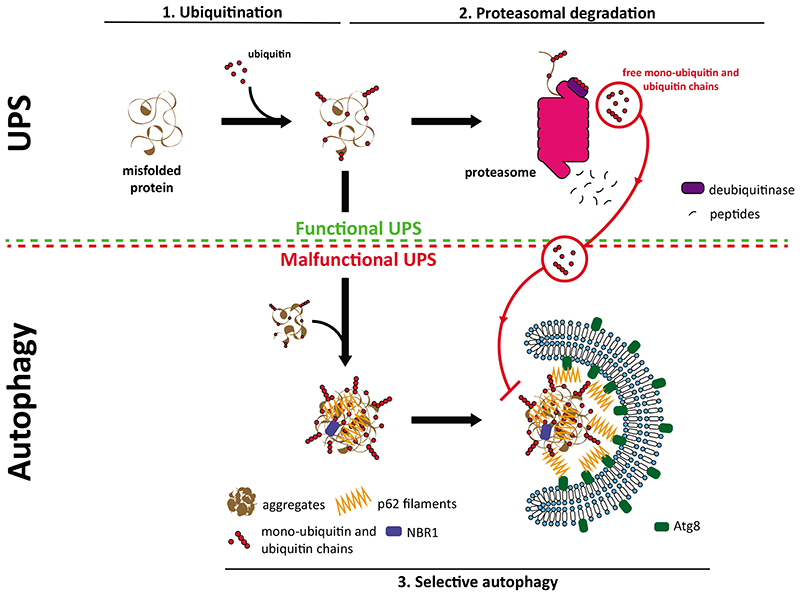
Fig. 3. Crosstalk between the UPS and selective autophagy.
In physiological conditions, misfolded and ubiquitinated proteins (1) are targeted mainly to the proteasome for their degradation (2). In response to proteotoxic stress and as consequence of proteasome overload or malfunction, misfolded proteins can no longer be degraded by the proteasome. In this situation, selective autophagy (3) serves as a backup mechanism and degrades these proteins. p62 filaments act at the intersection between the UPS and autophagy by sensing the accumulation of ubiquitinated substrates and mediate their phase separation into larger condensates. p62 oligomerization is a crucial step in this process and, in vitro, it is inhibited by free K48- and K63-linked ubiquitin chains. Free monoubiquitin also inhibits phase separation, likely by binding to the UBA domain of p62. Since free ubiquitin chains and monoubiquitin are generated by active proteasomes this provides a possible mechanism by which the selective autophagy of proteins and protein aggregates could be inhibited by the UPS.