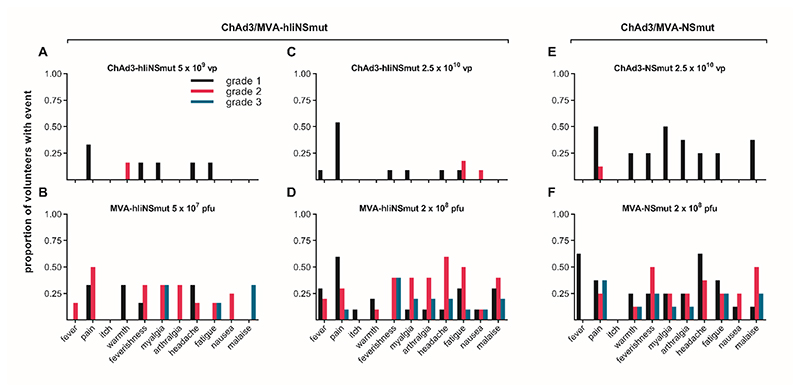
Figure 1. Solicited adverse events within 7 days from vaccination.
Frequency of local and systemic adverse events recorded by volunteers on diary cards. The proportion of volunteers reporting symptoms at any time during 72 hours following (A) Low dose: ChAd3-hIiNSmut (5 x10^9 vp) (n=6); (B) Low dose: MVA-hIiNSmut (5 x10^7 pfu) (n=6), (C) Standard dose: ChAd3-hIiNSmut (2.5 x10^10 vp) (n=11); (D) Standard dose: MVA-hIiNSmut (2 x10^8 pfu) (n=10); (E) Standard dose: ChAd3-NSmut (2.5 x10^10 vp) and (F) Standard dose: MVA-NSmut (2 x10^8 pfu). Color code indicates maximum severity of the reaction reported: black– Grade 1 (mild); red– Grade 2 (moderate); blue-Grade 3 (severe).