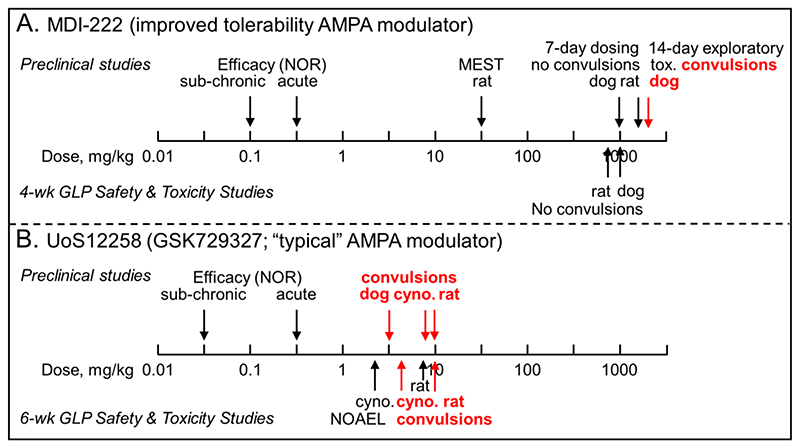
Figure 8. Comparison of the preclinical safety profiles showing the much safer profile of MDI-222 compared to UoS12258.
A. MDI-222 was not proconvulsant in the maximal electroshock threshold test (MEST) assay at a dose of 30 mg/kg p.o. and neither did it cause convulsions during 7-day dose ranging studies (maximum doses in rat and dog = 1,600 and 1,000 mg/kg p.o., respectively) or 4-week GLP Safety and Toxicity studies (maximum doses in rat and dog = 800 and 1,000 mg/kg p.o., respectively). To overcome solubility-limiting sub-proportional concentrations at higher doses, 14-day exploratory toxicity studies were conducted using a nanomilled formulation. In plasma drug concentrations equivalent to a dose of ~2,000 mg/kg of the standard formulation, convulsions were observed. B. UoS12258 was proconvulsant in the MEST assay at a dose of 10 mg/kg p.o. (data not shown for clarity; Ward et al., 2017) and during dose-range finding studies caused convulsions in dogs, cynomolgus (cyno.) monkeys and rats at doses of 3, 9 and 10 mg/kg p.o., respectively. Because of the sensitivity of dogs, the cynomolgus monkey was chosen as the second species, along with rat, for 6-week GLP Safety and Toxicity studies in which convulsions were observed at doses of 4 and 10 mg/kg in cynomolgus monkeys and rats, respectively with the no adverse effect levels (NOAEL) being 2.5 and 7.5 mg/kg, respectively. For both compounds, the efficacy assay used to define the therapeutic margin was the novel object recognition (NOR) assay. Doses shown are mg/kg p.o.