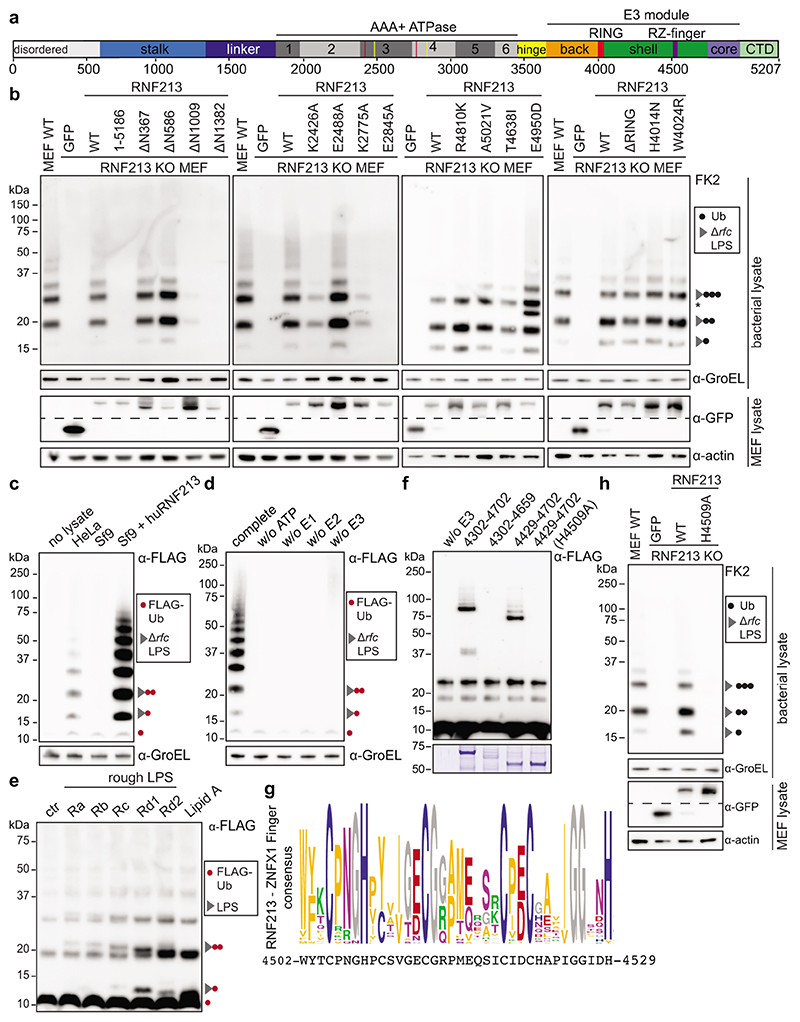
Figure 3. LPS ubiquitylation by RNF213 is a RING-independent, RZ finger-mediated reaction.
a, RNF213 domain structure. Critical residues in Walker A (K2426 and K2775) and Walker B motifs (E2488 and E2845) are indicated with red and yellow lines, respectively. RZ finger RNF213-ZNFX1 finger, CTD C-terminal domain. b, h, immunoblot analysis of S. Typhimurium Arfc extracted from WT or RNF213KO MEFs complemented with GFP or GFP-RNF213 alleles as indicated. GFP blots present the upper and lower part of continuous blots from which the middle parts have been removed as indicated by the dashed line. c-e, Immunoblot analysis of S.Typhimurium Arfc extracted from HeLa cells (c,d), or purified LPS from indicated S.Minnesota strains (e) and subjected to in vitro ubiquitylation using HeLa, Sf9 or Sf9 expressing huRNF213 lysates (c) or purified RNF213 (d,e). f, Immunoblot analysis of an IVU reaction using GST-tagged RNF213 fragments expressed in E. coli to assess autoubiquitylating activity. Representative of 3 experiments. b-f, h, Blots were probed with the indicated antibodies. Actin and GroEL, loading controls for mammalian and bacterial lysates respectively. g, RZ finger consensus amongst RNF213 and ZNFX1 proteins, human RNF213 protein sequence is depicted below. b, *, non-specific band. b,d-f,h, Representative of at least 3 biological repeats. c, n=1 For gel source data, see Supplementary Fig. 1.