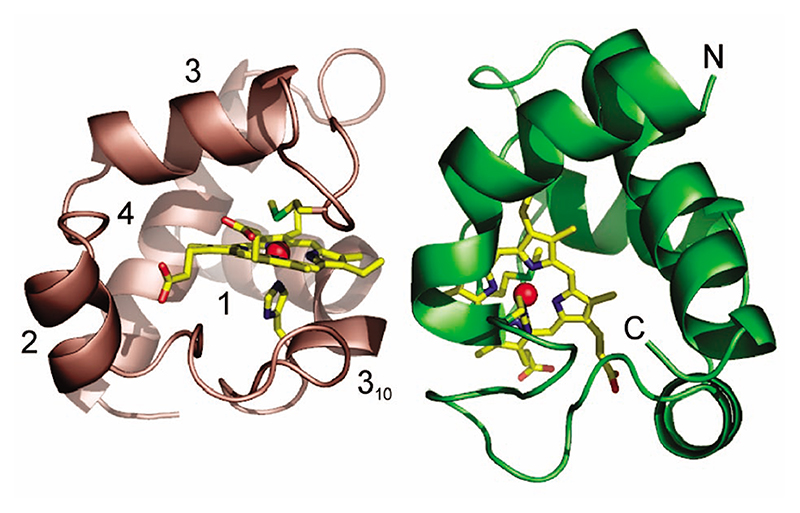
Figure 1. Three-dimensional structure of the homodimer of reduced P. laminosum cyt c 6.
Chains A and B are colored green and salmon, respectively. The heme and axial ligands are in stick representation with the Fe as a red sphere. Each monomer is composed of four α-helices, three β-turns, and one γ-inverse turn. Helix 1 is distorted at C15 with residues 15–18 part of a 310 helix fragment. The N and C terminal helices are indicated for chain A.