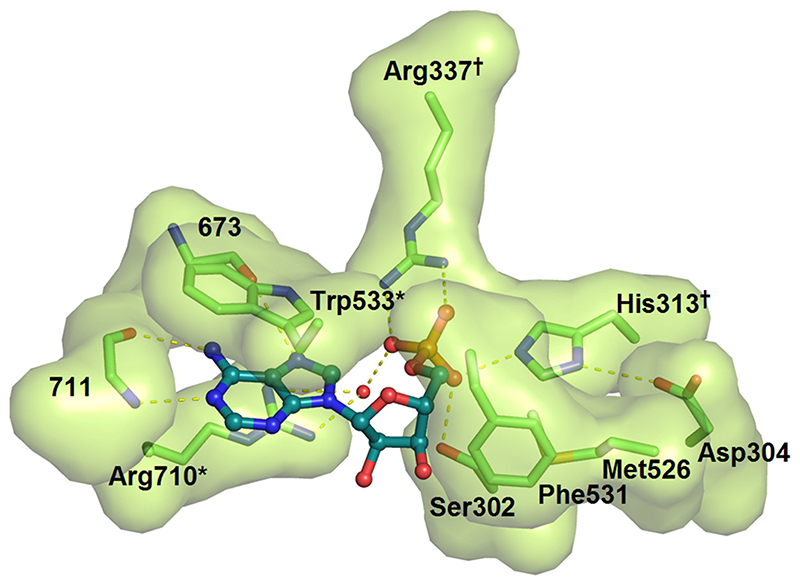
Figure 5.
The AMP binding pocket highlighting key residues that engage the ligand. The interacting residues are shown with a solvent-accessible surface. The adenine ring is base-stacked against the indole ring of Trp533 and the aliphatic portion of Arg710. Hydrogen bonds from the back-bone of residue 711 and 673 specify the adenine form of the purine ring. The phosphate of AMP forms a bidentate salt-bridge with Arg337, a water-mediated H-bond with Arg710, and H-bonds with Ser302 and His313. Two double Ala mutants were engineered to disrupt interactions with the adenine ring (*) and the phosphate (†) of AMP.