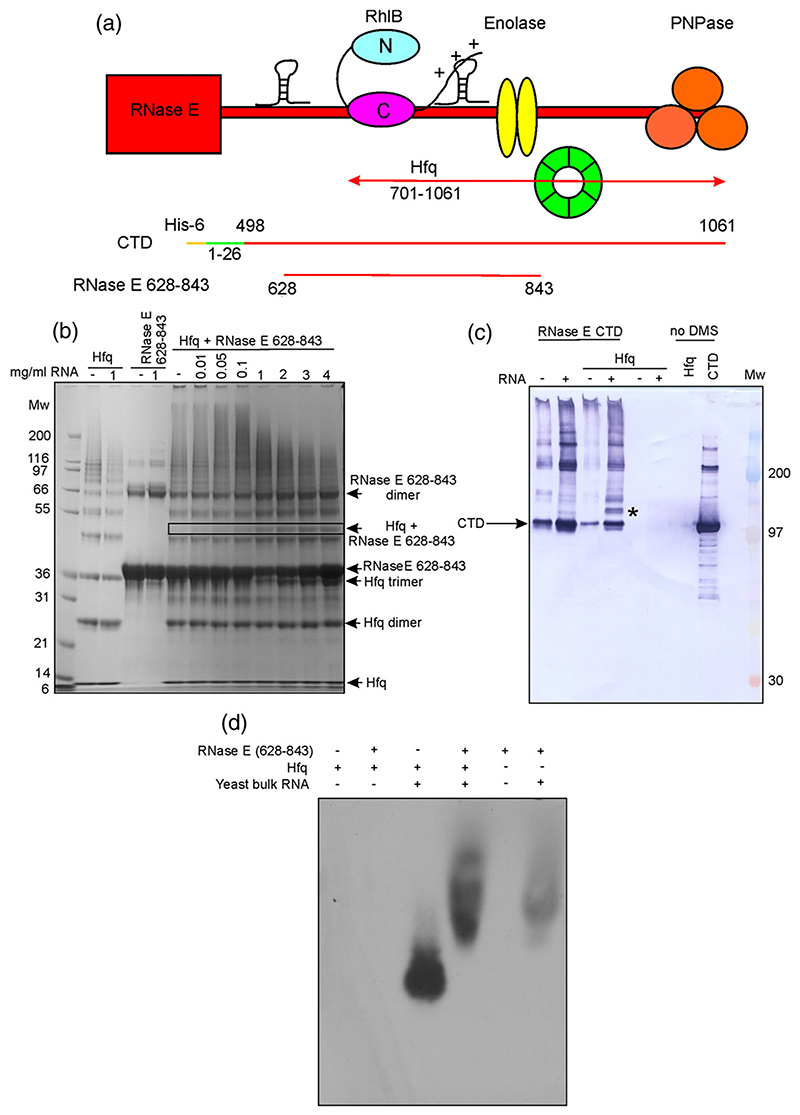
Fig. 6. The interaction between Hfq and RNase E is RNA-mediated.
(a) Schematic cartoon depicting the RNase E constructs used to investigate RNase E/Hfq interactions. Hfq (green) is reported to bind RNase E in the 701–1061 region.29 (b) SDS-PAGE analysis of Hfq and RNase E samples treated with the cross-linking reagent DMS. With increasing amounts of RNA, a new band (indicated by a box) attributed to a Hfq/RNase E(628–843)/RNA interaction appeared. Distribution of higher-molecular-weight species, which can be seen in the smeared pattern was also formed; this diminished with higher concentrations of RNA. Molecular weight markers (in kDa) are indicated. (c) Immunoblot against His-tagged RNase E CTD. Mixtures of RNase E CTD, Hfq, and bulk yeast RNA were treated with DMS and resolved by SDS-PAGE, followed by immunoblotting. A band is detected (*) when RNase E CTD is incubated with Hfq and RNA, but is not present if either Hfq or RNA is omitted, suggesting the formation of a ternary complex. (d) Immunoblot against Hfq, following native electrophoresis of mixtures of RNase E(628–843), Hfq, and bulk yeast RNA. In the presence of RNase E (628–843), the mobility of the Hfq/RNA complex is retarded (lane 4), suggesting an interaction between these components. The Hfq antibody cross-reacts weakly with the RNase E(628–843)/RNA complex, causing background labeling. Hfq does not enter the gel in the absence of RNA.