Fig. 4 |. CCTM-VbIc channels in DIBs.
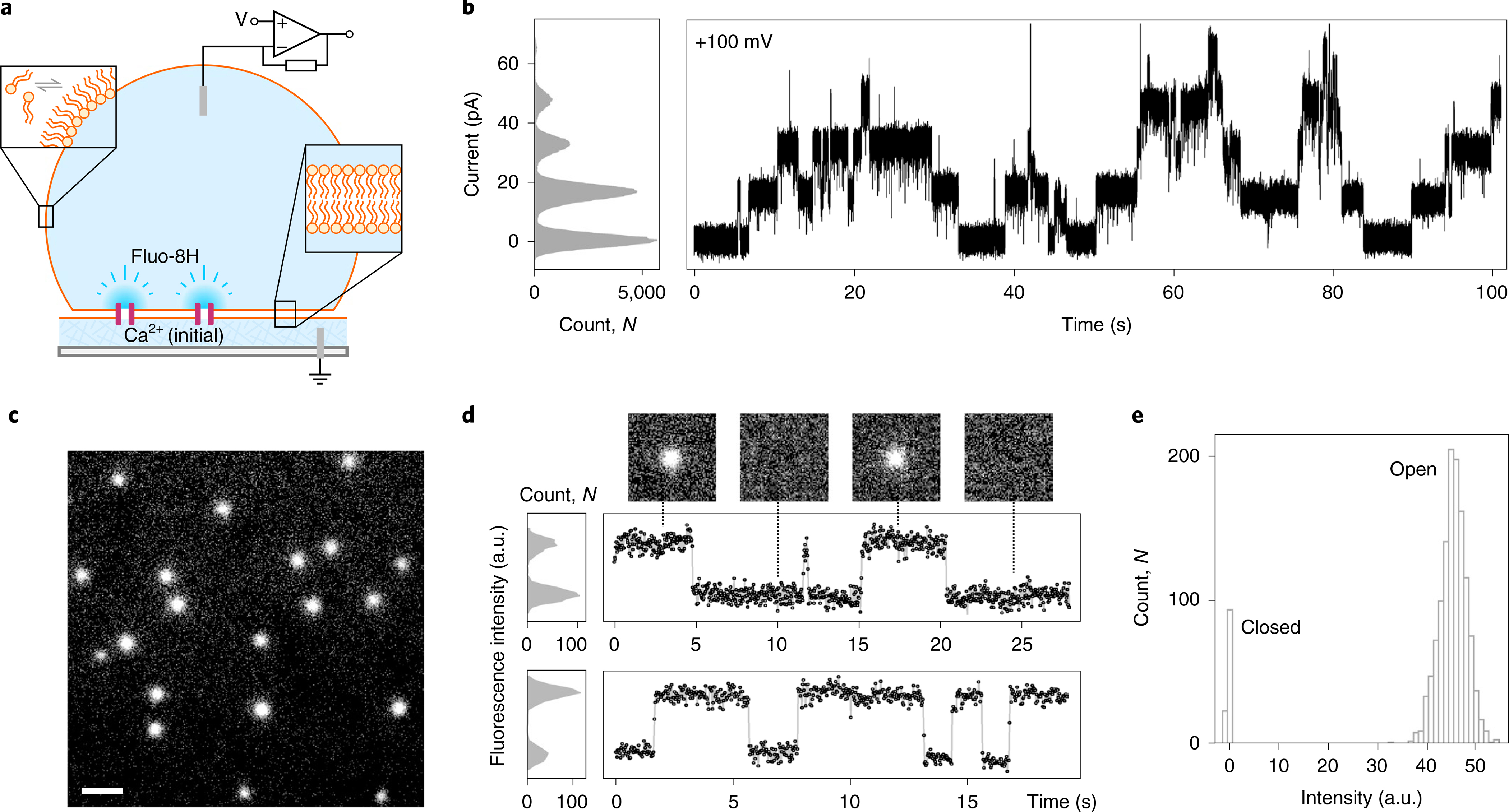
a, Schematic of a DIB formed between an aqueous droplet and a hydrogel substrate spun onto a coverslip (grey), both in the presence of a lipid-in-oil solution. The DIB has Ca2+ ions in the hydrogel and the Ca2+-sensitive dye Fluo-8H in the droplet. Channels (purple blocks) formed in the bilayer allow passage of Ca2+ ions into the droplet to generate plumes of Ca2+-bound dye (depicted in darker blue) that can be imaged using total internal reflection fluorescence microscopy. b, Conductance steps from multiple insertions of CCTM-VbIc in a DIB at +100 mV. Voltages are trans relative to cis (peptide). c, oSCR for CCTM-VbIc channels in a DPhPC membrane at +100 mV. This is a single 30 ms exposure; 100 nM CCTM-VbIc; scale bar, 10 μm. d, Fluorescence intensity versus time traces for two CCTM-VbIc channels. The y-axes represent mean pixel values of bilayer patches containing the channels. The upper trace is annotated with 30 ms frames (17.8 × 17.8 μm) from the respective oSCR image stack. e, Spot intensities extracted from eight CCTM-VbIc channels in the same bilayer, which represent 40 s of open-channel time, show a unitary open state and a closed state. For all experiments, the droplet peptide concentrations were between 25 and 100 nM.
