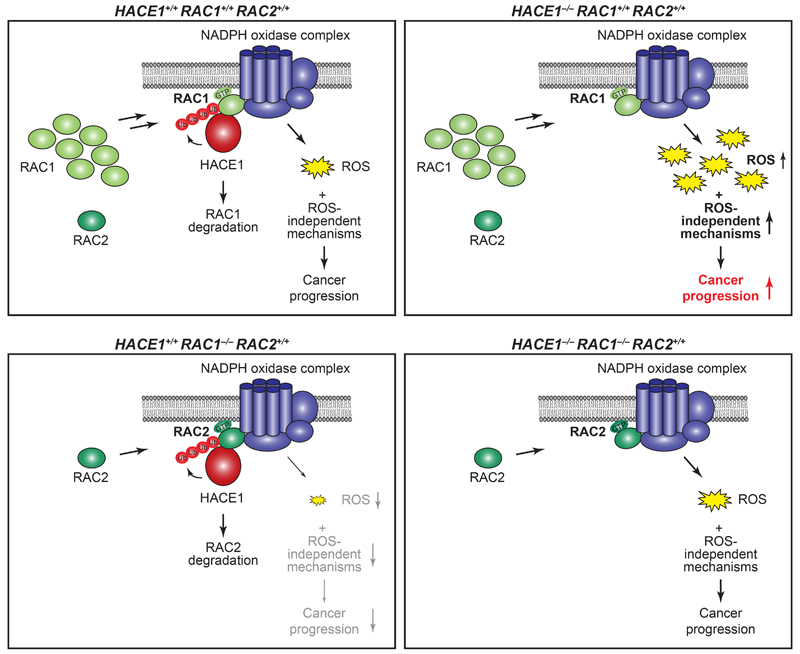
Figure 7. Schematic representation of HACE1 and RAC-family GTPases driving lung cancer development.
HACE1 ubiquitylates GTP-RAC1 when bound to the NADPH oxidase complex, leading to RAC1 degradation and thereby controlling ROS production (top, left). HACE1 deficiency results in an accumulation of GTP-bound RAC1, increased NADPH oxidase activity and enhanced levels of genotoxic cellular ROS, promoting cancer progression (top, right). Additionally, deregulated RAC1 could promote tumor development by ROS-independent mechanisms. In the absence of the more abundant RAC1, the activity of GTP-RAC2 when bound to the NADPH oxidase complex is controlled by HACE1, leading to decreased cellular ROS levels (bottom, left). When HACE1 and RAC1 are both ablated, active GTP-RAC2 can compensate and promote cancer progression (bottom, right).