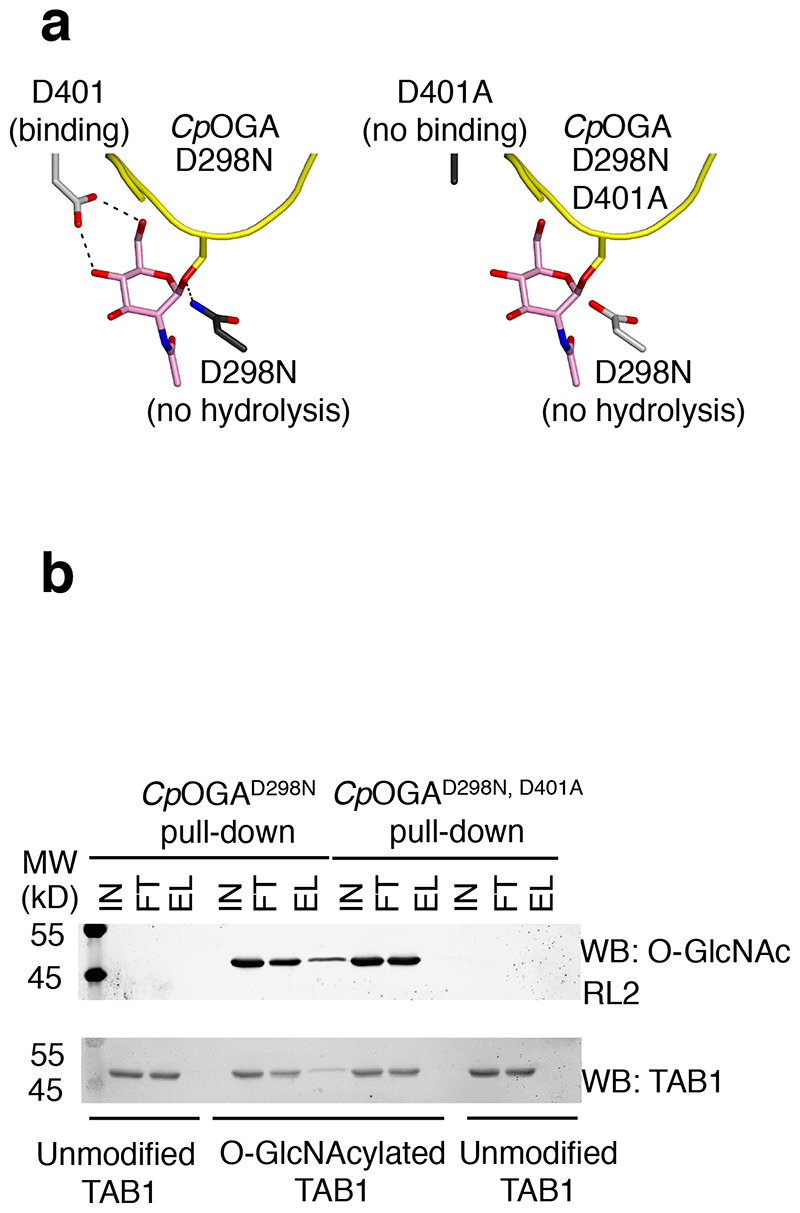
Figure 1. A point mutant of CpOGA can be exploited as a substrate trap for the enrichment of O-GlcNAcylated proteins.
(a) The inactive mutant CpOGAD298N can bind to substrate proteins (substrate is shown as a yellow cartoon, with GlcNAc depicted with pink sticks) but cannot hydrolyse GlcNAc therefore trapping O-GlcNAc modified proteins. The double mutant CpOGAD298N,D401A cannot bind O-GlcNAcylated proteins and therefore cannot act as a substrate trap
(b) Unmodified or O-GlcNAcylated TAB1 was incubated with Halo-CpOGAD298N coupled covalently to HaloLink beads. Pull down using the binding-deficient mutant CpOGAD298N,D401A was included to test the specificity of the pull down. Input, flow-through and elution fractions were blotted and probed with the antibodies mentioned. Elutions were performed by boiling the beads with sample buffer. TAB1 was pulled down in an O-GlcNAc specific manner by CpOGAD298N but not the control probe as evidenced by the presence of modified but not unmodified TAB1 in the elution fractions from CpOGAD298N.