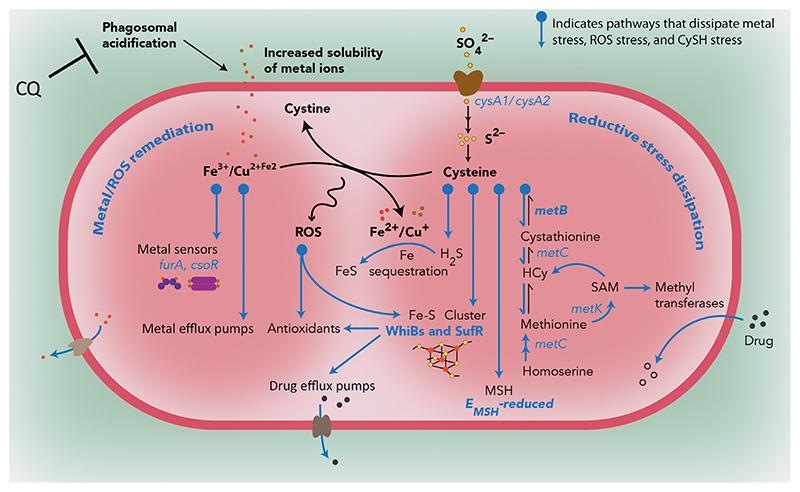
Figure 5. Redox-Altered Mycobacterium tuberculosis (Mtb) Inside Naïve Macrophages Acquires Drug Tolerance through Dissipation of Metal and Reductive Stress.
Exposure to acidic pH in macrophages leads to perturbation of redox homeostasis and the appearance of redox-altered populations of Mtb. Acidity in phagosomes induces the accumulation of soluble forms of metal ions (Fe/Cu) as well as the redox-active amino acid cysteine (CySH). The former can catalyze the generation of reactive oxygen species (ROS) by either driving the Fenton reaction or oxidizing CySH to cystine. Transcriptional profiling of intramacrophage Mtb fractions reveals that drug-tolerant EMSH-reduced Mtb are particularly efficient in dissipating reductive and metal stress during infection. This Mtb fraction channels the flux of CySH into the reverse transsulfuration pathway leading to H2S generation (MetB), Fe-S cluster biogenesis (SufR), and mycothiol (MSH) production. Induction of these pathways affords protection from oxidative stress by increased expression of redox- and acid-sensitive transcription factors (WhiB family and Suf system) that regulate Mtb’s antioxidant response, consume excess CySH and Fe, and induce the activity of metal and drug efflux pumps. Additionally, S-adenosylmethionine (SAM)-dependent methyltransferases are found to be highly expressed in reduced Mtb, where they can inactivate antibiotics by N-methylation. Blocking phagosomal acidification with lysosomotropic agents such as chloroquine (CQ) reverses intramacrophage redox heterogeneity and reduces isoniazid (Inh) or rifampicin (Rif) tolerance in Mtb-infected macrophages as well as in chronically infected animals. From [53], reprinted with permission from AAAS.