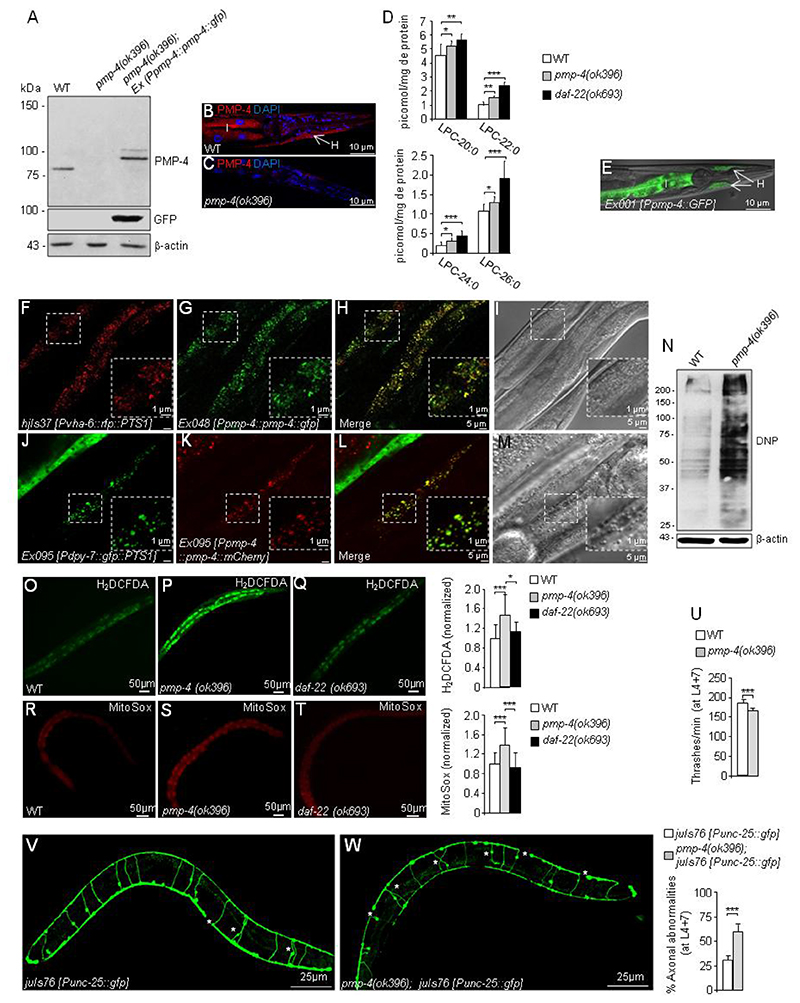
Figure 1. C. elegans pmp-4(ok396) mutants recapitulate the main hallmarks observed in X-ALD.
(A) PMP-4 and PMP-4::GFP protein levels in wild-type (WT), pmp-4(ok396) and pmp-4(ok396) animals expressing PMP-4::GFP under the control of the pmp-4 promoter (pmp-4(ok396); Ex042 [Ppmp-4::pmp-4::gfp]) at the L4 larval stage. β-actin was used as a loading control (bottom panel) (n=4 pools of worms by condition). (B-C) Immunofluorescence staining of formaldehyde-fixed (B) WT and (C) pmp-4(ok396) worms incubated with polyclonal anti-PMP-4 (red) and counterstained with DAPI (blue) at the L4 larval stage. The posterior part of the worm is depicted. I=intestine and H=hypodermis. Scale = 10μm. (D) Lysophosphatidylcholine fatty acid levels (LPC-20:0, LPC-22:0, LPC-24:0 and LPC-26:0) of L4 worm lysates of WT (n=12), pmp-4(ok396)(n=10) and daf-22(ok693) animals (n=6). The daf-22(ok693) mutant was used as a positive control. (E) Tissular expression of GFP under the control of the pmp-4 promoter (Ex001 [Ppmp-4::gfp]) in the intestine=I and hypodermis=H at L4. The worm is oriented head to the left, dorsal face-up. The same results were obtained by three independent transgenic lines. Scale = 10μm. (F-I) The construct containing PMP-4::GFP under the control of the pmp-4 promoter was injected into the strain labelling peroxisomes with RFP in the intestine (hjIs37 [Pvha-6p::rfp::PTS1]; Ex048 [Ppmp-4::pmp-4::gfp]). Images of (F) RFP::PTS1 and (G) PMP-4::GFP staining. (H) Merge of images F and G. In yellow, colocalization of RFP::PTS-1 with PMP-4::GFP. (I) DIC-Nomarsky pictures corresponding to the fluorescence images (n=30). (J-M) The constructs containing GFP::PTS1 under the control of a specific hypodermal promoter (Pdpy-7) and PMP-4::mCherry under the control of the pmp-4 promoter were coinjected in the N2 strain (Ex095 [Pdpy-7::gfp::PTS1 + Ppmp-4::pmp-4::mCherry]). Here, the peroxisomes are labelled with GFP in the hypodermis. Images of (J) GFP::PTS1 and (K) PMP-4::mCherry staining. (L) Merge of images J and K. In yellow, colocalization of GFP::PTS-1 with PMP-4::mCherry. (M) DIC-Nomarsky images corresponding to the fluorescence images (n=30). Scale = 5 μm in each figure except for the expanded pictures in the squares, in which scale = 1 μm. The worms in the figures are oriented head to the left, dorsal face-up and only a portion of the central part of the body is depicted. (N) Dinitrophenol (DNP) protein levels in WT and pmp-4(ok396) animals at the L4 larval stage. The quantification of these blots by densitometry was performed and normalized to β-actin (n=4 pools of worms by condition). Relative total ROS levels (H2DCFDA) were measured by quantifying the fluorescence emission of the H2DCFDA probes in living animals in (O) WT (n=51), (P) pmp-4(ok396)(n=66) and (Q) daf-22(ok693) (n=40) L4 nematodes maintained in a liquid medium. Values are normalized to WT. Relative mitochondrial ROS levels in living animals in (R) WT (n=66), (S) pmp-4(ok396) (n=88) and (T) daf-22(ok693) (n=66) L4 nematodes quantified with MitoSox probes. Values are normalized to WT worms. Scale = 50 μm. (U) The thrashing behaviour of WT and pmp-4(ok396) nematodes was analysed in liquid medium at L4+7 days (n=20 animals/condition). Representative fluorescence images of GFP-labelled GABAergic neurons showing axonal abnormalities and quantitative analysis in living worms in (V) juIs76 [Punc-25::gfp] (n=20) and (W) pmp-4(ok396); juIs76 [Punc-25::gfp] (n=20) at L4+7 days. Scale = 25 μm. Data represent the mean ± standard deviation (SD). Statistical analysis was carried out with one-way ANOVA, followed by Tukey’s post hoc test (*P<0.05; **P<0.01; ***P<0.001) for D, O-S. Statistical analysis was carried out with Student’s t-test (*P<0.05; **P<0.01; ***P<0.001) for U-W.