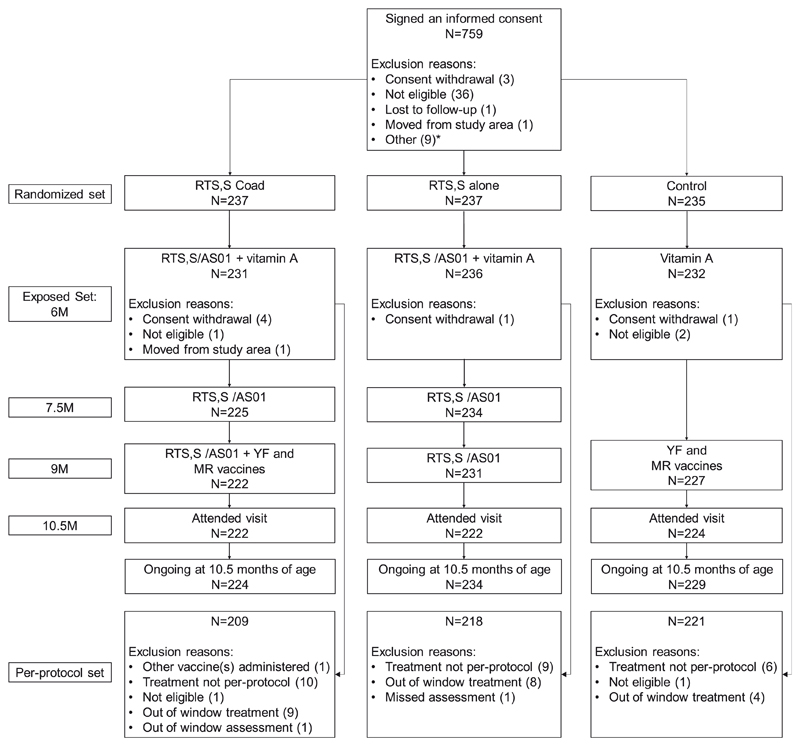
Fig. 3.
Flow of participants. The RTS,S coad group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age and the YF and MR vaccines at 9 months of age; the RTS,S alone group received the RTS,S/AS01 vaccines at 6, 7.5 and 9 months of age; and the Control group received the YF and MR vaccines at 9 months of age. N, number of children, M, month; RTS,S/AS01, pre-erythrocytic Plasmodium falciparum malaria vaccine; YF, yellow fever; MR, combined measles-rubella. *An informed consent form was signed for 9 children when the enrolment target was met and these children were not included in the study.