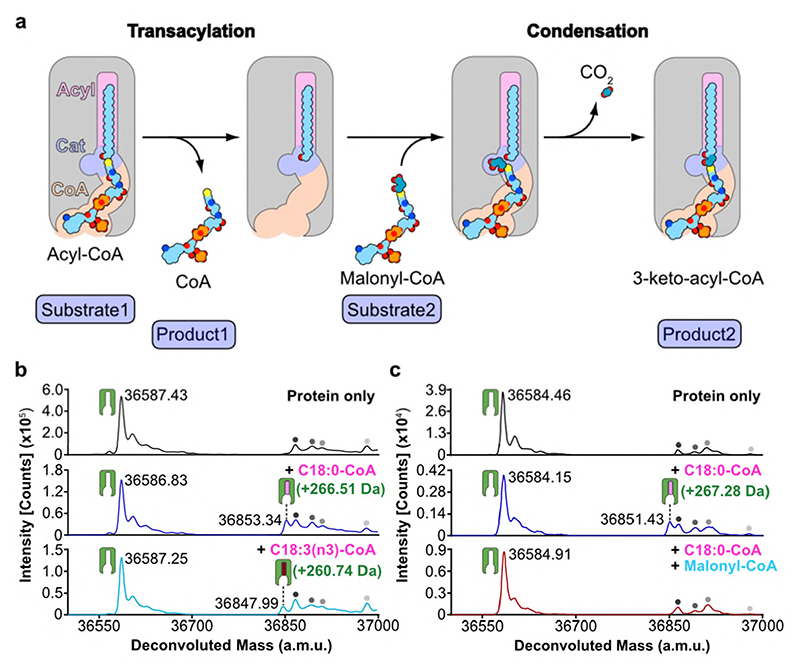
Fig. 5. Proposed ping-pong mechanism and evidence for a covalent acyl-enzyme intermediate.
a, Schematic outlining proposed ELOVL ping-pong mechanism. b-c, Intact mass analysis of ELOVL7 in presence of b, acyl-CoA substrates demonstrating transacylation to form an acyl-enzyme intermediate and c, acyl-CoA followed by malonyl-CoA. Intact mass analysis of protein only (black), protein + C18:0 acyl-CoA (blue), protein + C18:3 acyl-CoA (cyan) and protein + C18:0 acyl-CoA + malonyl-CoA (red). Peaks are indicated as follows: protein (green icon), acyl enzyme intermediate (green icon + pink/red oblong), background species present in all traces (grey circles). (n=2 biological repeats, see Supplementary Figures 1-2 for replicate traces; Supplementary Table 2 for experimental and theoretical masses and mass errors).