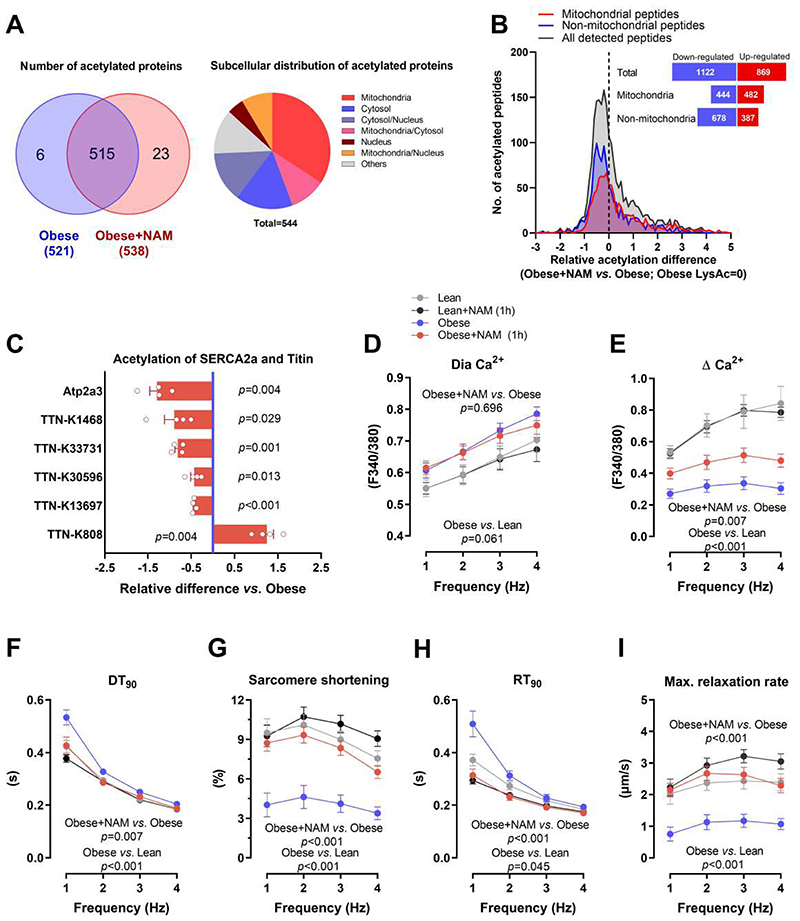
Fig. 4. SERCA2a deacetylation contributes to improved diastolic function by NAM.
(A) Venn diagram (left) showing the overlap of detected acetylated proteins in the hearts of control and NAM-treated 20-week-old male ZSF1 obese rats, along with their subcellular localization in a pie chart (right). (B) Distribution of NAM-induced changes in acetylation of cardiac peptides (mitochondrial and non-mitochondrial); the dashed line denotes acetylation in control ZSF1 obese rats. The inset figure denotes the sum of peptides with up- or down-regulated acetylation. (C) Relative signal intensity difference of significantly regulated acetylation sites in titin and SERCA2a (Atp2a3). Note that negative values indicate deacetylation and positive ones indicate acetylation, (n=4 obese+NAM compared to the average of 3 obese). (D) Diastolic (Dia) calcium and (E) changes in calcium transient amplitude as indicated by Fura-2/AM ratio (340:380 nm), (F) time to 90% decay (DT90), along with (G) simultaneously measured sarcomere shortening, (H) time to 90% relaxation (RT90) and (I) maximal relaxation rate of electrically-paced adult ZSF1 obese and lean cardiomyocytes that were pre-incubated or not with NAM (100 μM) for one hour, (n=18/20/18/23 cardiomyocytes in lean/lean+NAM/obese/obese+NAM isolated from 4 lean and 4 obese ZSF1 rats at the age of 20 weeks). P values were calculated by (C) one-sample t-test or (D to I) two-way repeated measures ANOVA. Bars/circles and error bars show means and SEM, respectively, with individual data points superimposed.