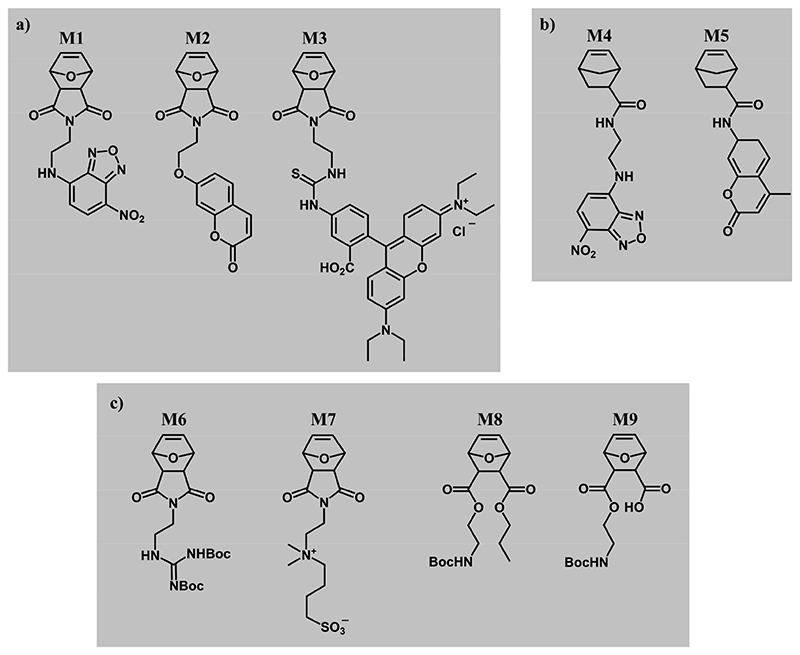
Figure 2. ROMP monomers for bioactivity studies.
a) Fluorescently labeled oxanorbornene-imide monomers M1, M2 and M3 with nitrobenzofurazan (NBD), coumarin (COU), Rhodamine B (RHB) fluorophores, respectively. b) Fluorescently labeled norbornene-amidate monomers M4 and M5 with NBD and COU fluorophores, respectively. The fluorescent monomers in a) and b) were copolymerized with structurally similar bioactive ROMP monomers c): the guanidinium oxanorbornene M6, the sulfobetaine monomer M7, the propyl-oxanorbornene M8, and the protected zwitterionic monomer M9. All monomers have exo-configuration.