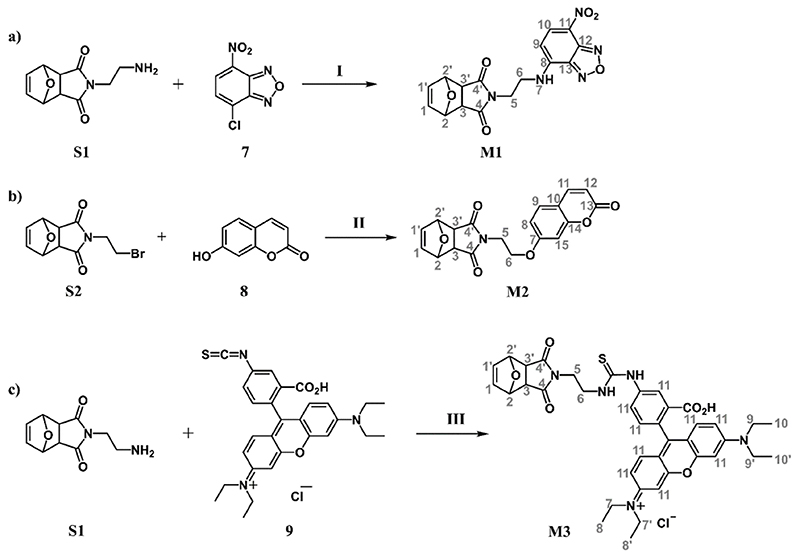
Figure 3. Synthesis and NMR peak assignment of fluorescent oxanorbornene imide monomers M1, M2 and M3.
a) Green-fluorescing NBD-monomer M1 (I: CH2Cl2, diisopropylethylamine); b) blue-fluorescing COU-monomer M2 (II: acetone, K2CO3); c) red-fluorescing RHB-monomer M3 (III: MeOH). The numbering of atoms of the products refers to the peak assignments in the Experimental.