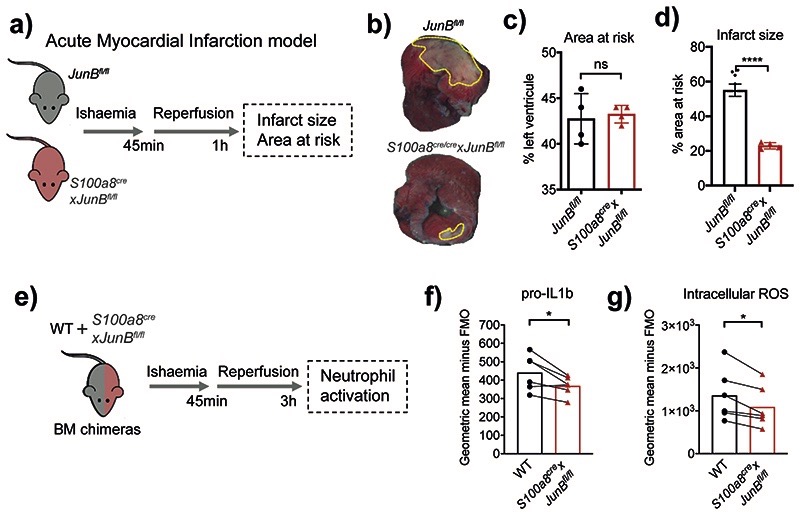
Fig. 7. Neutrophil-specific knock-out of JunB limits pathological destruction of tissue during neutrophil-driven inflammation.
a) Mouse model of acute myocardial infarction. The left anterior descending coronary artery is occluded for 45 min followed by reperfusion for 1 h.
b) Representative images of heart slices stained with triphenyltetrazolium chloride and normalized to area at risk (negative Evans blue staining) from JunBfl/fl and S100a8cre/crexJunBfl/fl mice subjected to ischemia reperfusion. Dotted yellow lines highlight areas of dead myocardium.
c, d) Histological evaluation of left ventricle area at risk (C) and infarct size (D) in mice subjected to ischemia reperfusion. All results are shown as means and SD derived from four mice from each group within one experiment. Significant differences compared between JunBfl/fl and S100a8cre/crexJunBfl/fl mice was determined by unpaired student t test analysis. ns, not statistically significant; *p < 0.05; ****p < 0.0001.
e) Mixed bone marrow chimera setup to measure neutrophil activation in shared inflammatory environment of acute myocardial infarction.
e, g) Production of pro-IL1b (e) or ROS (g), as measured by flow cytometry in WT and JunB-deficient neutrophils in mixed bone marrow chimera mice, subjected to permanent myocardial infarction. Data are shown as means and SD derived from three mice from each group within one experiment. Statistical comparison was made by paired student-t test; *p<0.05.