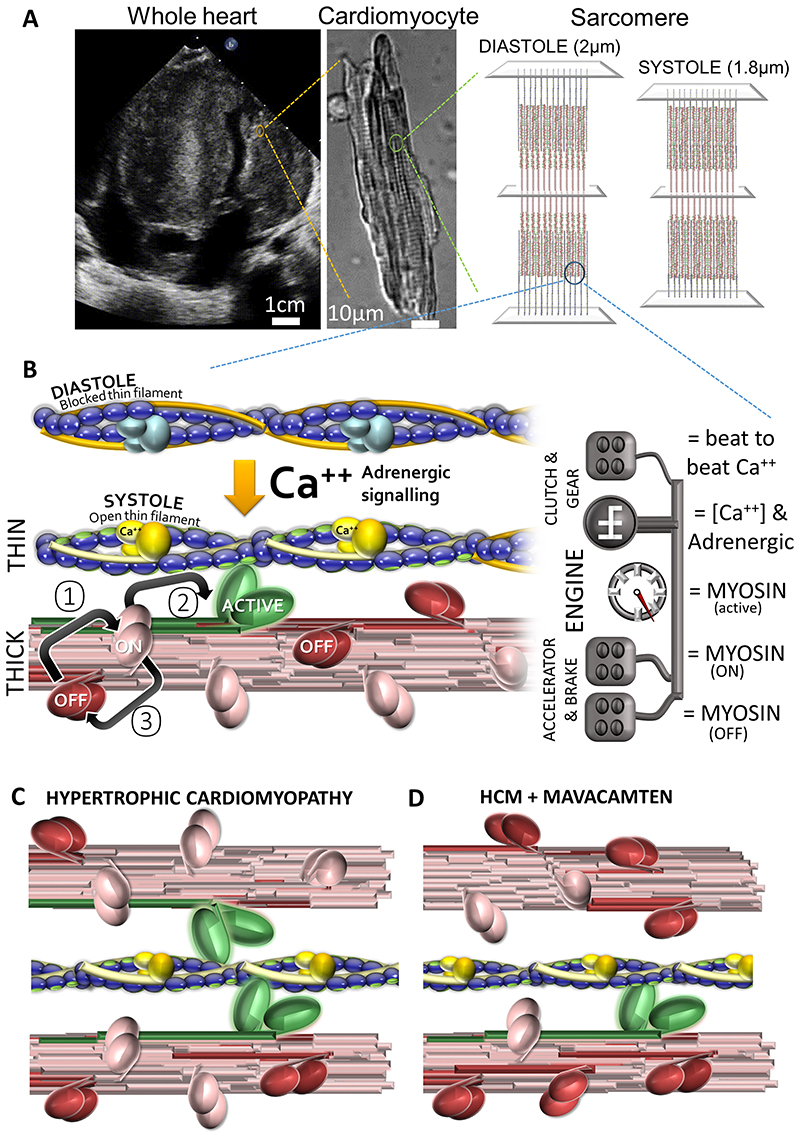
Figure 1. Myofilament activation and modulation.
(A) Contractility of the whole heart, in this case a heart with hypertrophic cardiomyopathy and massive septal thickening, arises from the coordinated activation of myosin motors organised into regular sarcomere arrays in single cardiomyocytes. (B) Force production is controlled by regulating myosin activity. Actin stimulates the myosin ATPase. Actin availability is controlled by fluctuating calcium levels which via the troponin complex in the thin filament regulate a molecular clutch shielding actin (purple balls) from myosin in low diastolic calcium (Ca++) and exposing it in high systolic Ca++. Myosin is bundled, forming the thick filament. The exposed head contains the motor domain which cycles between “super-relaxed” OFF (red), “relaxed” ON (pink) and “Active” force producing states (green). In diastole the OFF state predominates. In systole as the myofilament activates, an intrinsic thick filament mechanism facilitates the transition between OFF and ON (①). Peak contractility occurs with 1:10 myosin heads active. Small molecule modulators of this system are in clinical evaluation. Omecamtiv mecarbil, a Myosin ATPase activator, increases force production by stimulating transition ②. Mavacamten, a myosin ATPase inhibitor, stabilises the super-relaxed OFF state ③. (C) Hypertrophic cardiomyopathy-causing variants typically reduce myosin locked in the OFF state. With more myosin active, force production in systole and diastole increases (more green, fewer red myosin heads). (D) Restoring the balance of myosin activation in HCM, using myosin ATPase inhibitors like mavacamten, reduces force production (and ATP consumption) in systole and diastole (more red, less green).