Abstract
Surviving in natural environments requires animals to sense sudden events and swiftly adapt behaviour accordingly. The study of such Reactive Adaptive Behaviour (RAB) has been central to a number of research streams, all orbiting around movement science but progressing in parallel, with little cross-field fertilization. We provide a concise review of these research streams, independently describing four types of RAB: (i) cortico-muscular resonance, (ii) stimulus locked response, (iii) online motor correction and (iv) action stopping. We highlight remarkable similarities across these four RABs, suggesting that they might be subserved by the same neural mechanism, and propose directions for future research on this topic.
Keywords: Electromyography (EMG), Electroencephalography (EEG), Movement, Sensorimotor processing, Saliency, Surprise
Introduction
In the animal world, movement and life go hand in hand: an animal not able to move effectively is less likely to survive. Yet, the constraints posed by living in a rapidly-changing environment have pushed brains to evolve not only a sophisticated motor system, but also a tight coupling between movement and sensory encoding. Development of the motor system is indeed guided by perception. Likewise, perception alone does not develop properly without movement (1).
Out of the myriad of examples of sensory-motor integrative processes only some are subject to volition – the individual’s ability to choose whether or not to act in a given circumstance (2). For instance, a monkey actively looking for food might deliberately choose to climb a particular tree when it sees lots of fruits hanging on it. Yet, a sudden and unexpected change in the sensory scene might trigger an unavoidable reactive behaviour, having higher priority compared to the initial goal to collect food. A rustling coming from the branches above the monkey might lead the animal to jump out of the tree. The same sound coming from the ground might instead lead the monkey to climb even higher. Either of these clearly distinct actions – climbing or jumping off the tree – albeit appropriately chosen on the basis of the context, take often place with none or scarce influence of the animal’s volition. Yet, either is indispensable for survival.
Here we refer to this way of acting (or modulation of acting) as “Reactive Adaptive Behaviour” (RAB). RAB falls in the nexus between reflexive and volitional movements. Similarly to reflexes, RAB is reactive and therefore stimulus-driven. However, similarly to voluntary actions, RAB is adaptive, i.e. flexible to the ever-changing nature of the environment. As such, RAB questions the very dichotomy between reflexes and voluntary actions (3, 4), calling for a reflexive-volitional gradient wherein RAB itself lies.
We highlight four fundamental features that apply to RAB. First, RAB is evoked by sudden and unexpected changes in the sensory scene, i.e. by “salient” stimuli. Second, RAB is characterized by its short-latency: it is rapid, and elicited in situations where speedy responses can be vital. Third, RAB is adaptive, i.e. flexible on the basis of the context: it favours those behaviours (e.g. climbing vs. jumping, according to the above example) that ensure survival and, in the long term, maximise fitness. This implies that RAB is the result of relatively complex neural computations, selecting motor output on the basis of the current environmental context. Fourth, as already anticipated above, RAB takes place with none or scarce influence of a subject’s volition.
Considering the above features, it turns out that RAB has been studied across a wide range of disciplines concerned with studying biological movement, such as neuroscience, psychology and biomechanics. Over the past couple of decades, a number of original observations have characterized specific manifestations of RAB using distinct experimental paradigms. Yet, we were stunned to realise that these research streams are largely progressing in parallel, with little cross-field fertilization. This prompted us to conceive the current work. We aim to provide a concise review of some research streams independently describing four particular RABs: (i) Cortico-Muscular Resonance, (ii) Stimulus Locked Response, (iii) Online Motor Correction and (iv) Action Stopping. While doing so, we highlight how each of these behaviours fulfils the above-described criteria for RAB, and discuss the possibility that they could be partly subserved by the same neural mechanism.
[i]. Cortico-Muscular Resonance (CMR)
The term Cortico-Muscular Resonance (CMR) has been recently proposed to refer to a series of fast modulations of muscular activity (and ensuing applied force) evoked by sudden sensory stimuli, irrespectively of their sensory modality (5, 6).
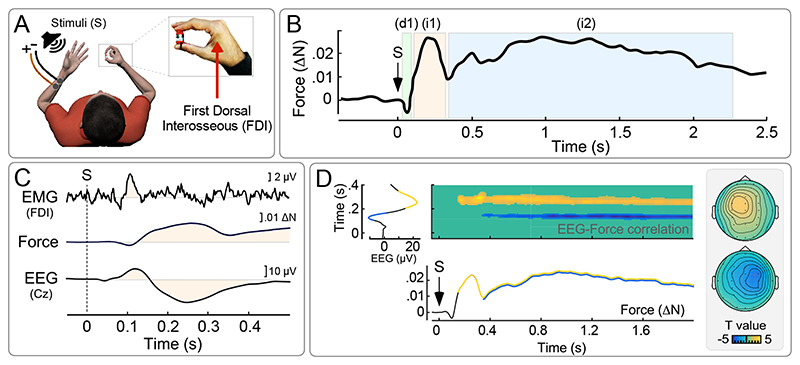
CMR has been observed using both electromyography (EMG) and force measurements. In a typical experiment, participants are required to exert a weak and constant isometric force on a transducer held between the index finger and thumb, while simple, task-irrelevant and fast-rising sensory stimuli (either auditory or somatosensory) are delivered (5, 6) (Fig. 1a). These stimuli evoke a multiphasic modulation of the exerted force: An initial transient force decrease (d1), peaking ~100 ms post-stimulus, is followed by a transient force increase (i1) peaking at ~250 ms, and by a second (longer lasting) force increase (i2) starting ~300-350 ms and lasting for ~2 seconds (Fig 2b). The two initial force modulations – d1 and i1 – have an EMG counterpart, detected when recording from the first dorsal interosseous muscle (FDI; i.e., the muscle contributing to the force exerted on the transducer using the index finger, Fig 1a). Expectedly, the EMG modulations have shorter latencies (~75 and ~110 ms, respectively) compared to the corresponding force modulations, due to the well-known electromechanical delay of motion with respect to muscular activity (Fig. 1c, Box 1).
Figure 1. Cortico-Muscular Resonance (CMR).
(A) Participants exert a weak and constant isometric force holding a transducer between the right index finger and thumb (~1 N). Both force and EMG (from the First Dorsal Interosseous, FDI) are simultaneously recorded. Somatosensory stimuli are delivered through electrical stimulation of the left median nerve or auditory stimuli are delivered through a loudspeaker placed nearby the left hand. (B) These fast-rising stimuli, regardless of sensory modality, elicit a multiphasic modulation of the exerted force, consisting of an initial decrease (d1, peaking ~100 ms post-stimulus), followed by a first transient increase (i1, peaking ~250 ms post-stimulus) and a second more tonic increase (i2, starting ~350 ms post-stimulus). (C) Simultaneous measurements of EMG activity (from FDI), Force, and EEG (at Cz): Signal modulations that co-vary across measurements are highlighted – see (5) for details. Time 0 indicates stimulus onset (S). Note that Force recordings lag behind EMG due to the well-known electromechanical delay of motion with respect to muscular activity (Box 1). (D) Trial-by-trial correlations between all timepoints of simultaneously collected EEG and Force modulations in response to the same somatosensory stimulation. Both i1 and i2 correlate with a widespread EEG positivity contralateral to the hand exerting the force (yellow, top scalpmap). Additionally, i2 correlates with an EEG negativity contralateral to the stimulated hand (blue, bottom scalpmap). Adapted with permission from (5).
Figure 2. Stimulus Locked Response (SLR).
(A) Paradigm used to study SLR in monkeys: The animals are trained to look at a central fixation point (FP). After a variable amount of time, the FP disappears, and the monkeys have to look to a new suddenly appearing peripheral target (PT). Intramuscular EMG activity is measured from several neck muscles including the obliquus capitis inferior (OCI), which subserves the rotation of the head towards the target. (B) The PT evokes in OCI a first transient burst of muscular activity (pink area), followed by a second period of tonic activity lasting until the saccade onset (light blue area). The magnitude of the transient burst predicted the latency of the following saccade. Adapted from (13). (C) Even when the animals do not need to produce a saccade (i.e. after the cue), the stimulus evokes the same pattern comprising two distinct phases. Adapted from (18). (D) Paradigm used to study SLR in humans: Participants move their arm under a non-transparent screen (shown in opaque in the figure for illustrative purposes), while only the visual feedback of the hand position is provided as a coloured dot. They are instructed to reach for the PT when this appears. Intramuscular EMG is recorded from a number of shoulder and arm muscles, including the deltoid posterior (DP) and the pectoralis major (PM). (E) PT appearance elicits a transient EMG burst (pink area), followed by the EMG activity consequent to the actual voluntary movement (light blue area). The magnitude of the first burst predicted the latency of the subsequent voluntary movement. Adapted from (12). (F) SLR implies a force useful for reaching the PT, even if participants are instructed to reach a location diametrically opposite to the PT. Adapted from (25).
BOX 1. Response latency and its sources of variability.
Several analogies across distinct RABs can be made on the basis of their response latency. Yet, caution must be exerted when doing so, considering some important physiological sources of latency variability. We discuss these below.
Measure types
The earliest indices of peripheral motor activity are electromyographic (EMG) responses reflecting the electrical activity produced by skeletal muscles. These EMG responses are followed by changes in force, either isometric or entailing actual movements (i.e. kinematics), both having a considerable electromechanical delay (30-60 ms) with respect to their underlying EMG activity (90, 91). Even longer latencies are observed when RABs are studied measuring response times (RTs; such as pressing a button), as their latency reflects the final stage of a movement (25, 92).
Sensory Modalities
The sensory modality of the stimuli used to elicit different RABs is another important factor to consider when comparing latencies across different studies. Cross-modal differences in both transduction and transmission times account for remarkable differences in response latency. It is difficult to precisely quantify these delays, as they also depend on the stimulus properties (see next point). Yet, using the peak latency of first negative wave of the supramodal and saliency-dependent EEG vertex potential as guidance (10), auditory stimuli yield the fastest responses, while responses to somatosensory stimuli delivered to the hand are slower by ~25 ms, and those to visual stimuli follow with an additional ~40 ms delay. This might contribute to some of the ostensive differences among RABs elicited by stimuli belonging to different sensory modalities.
Stimulus properties
Response latency also depends on the physical properties of the sensory stimulus, such as its magnitude and rise-time (93, 94). This generalizes well across different sensory modalities (93). In addition, when eliciting RAB using somatosensory stimuli, it is also imperative to consider what body part is stimulated and, most importantly, what type of stimuli are delivered. Indeed, different somatosensory stimuli can activate entirely different receptor classes, associated with different nerve fibres having different axon diameters, hence conduction velocities (95, 96).
Measured muscles
When measuring EMG (or related force/kinematic) activity, another factor causing differences in response latency is the proximity between the innervated muscle and the central nervous system. Considering the conduction velocity of both corticospinal tracts and motoneurons, the different travelling length can result in up to 20 ms in relatively extreme cases, e.g. when latencies of responses in the upper arm and the lower leg muscles are considered (97).
Onset vs. Peak latency
Another factor significantly affecting latency comparison is whether the onset or the peak of a response is measured. While peak latency is relatively straightforward to calculate (at least when dealing with transient responses), onset latency is more difficult to measure, and it is often estimated computing the response’s first-order derivative. Although onset and peak latencies are clearly correlated with one another, they sometimes have different predictive power (69). For instance, onset latencies can give more reliable estimates of neurophysiological properties such as conduction velocities of peripheral afferents (98, 99).
RAB-related neural measures
Just as the various measures of motor activity discussed above (EMG, force, RT), also the indices of stimulus-evoked neural activity can have different temporal profiles and considerable latency variability. Invasive recordings such as single and multi neuron activity sometimes yield earlier latencies compared to local field potentials (100). In turn, the EEG signal, which is recorded non-invasively from the skull, often depends on the simultaneous activity of multiple generators and therefore can display significant latency differences compared to local field potentials (80, 101). When examining the relationship between motor and neural measures these sources of latency variability should also be taken into account.
A few CMR features are worth being highlighted, as they nicely dovetail the features defining RAB (discussed in the previous section). First, CMR appears to be scarcely accessible to volition: not only participants are not meant to move in response to the stimuli, but they were mostly unaware of the modulation of their force output. Second, the CMR magnitude is considerably reduced when the eliciting stimulus has low behavioural relevance, e.g. when it is highly predictable (5). This observation highlights the adaptive character of the CMR, which is adjusted on the basis of the context, and preferentially triggered in response to stimuli that are more likely to require a swift reaction. Another feature that we highlight is that the CMR pattern evoked by auditory and somatosensory stimuli is extremely similar, indicating that CMR is consequent to a supra-modal neural mechanism. Notably, some research has described CMR-like modulations using also visual stimuli (7, 8).
The neural origin of CMR was explored using EEG recordings. It was observed that the stimuli eliciting CMR also evoke a concomitant Event Related Potential (ERP), dominated by two large negative-positive waves maximal at the scalp vertex (and therefore called ‘vertex potential’ (9, 10)) (Fig 1c). Like CMR, this ERP is evoked irrespectively of the modality of the stimulus, and its amplitude is reduced when the stimulus is highly expected (10, 11). Importantly, trial-by-trial analysis of simultaneous EEG-force recordings showed that the ERP and the CMR are tightly coupled: brain activity measured above the motor cortex contralateral to the hand exerting the force predicts the magnitude of i1 and i2. Furthermore, brain activity measured contra-laterally to the hand receiving a somatosensory stimulus predicts the magnitude of i2 (Fig. 1d). All together, this suggests that CMR originates from the effect of the saliency-induced vertex potential on the activity of specific cortical modules engaged in a certain task, including the corticospinal drive arising from frontal premotor/motor areas during the exertion of isometric force (5, 6).
[ii]. Stimulus Locked Response (SLR)
The term Stimulus Locked Response (SLR) has been coined to indicate short latency modulations of EMG activity evoked by sudden visual stimuli (12). These responses are typically recorded using intra-muscular EMG from neck and/or shoulder muscles of either human or non-human primates (12–14). SLRs exhibit a number of features typical of RAB, as discussed below.
[2.1]. SLRs in non-human primates’ neck muscles during saccade tasks
SLRs were first observed by Corneil et al., (2004) in monkeys performing a saccade task (Fig. 2a) [(13) task adapted from (15)]. Animals were trained to look at a central fixation point (FP). After a variable amount of time, the FP would disappear, and the monkeys had to look to a suddenly-appearing new peripheral target (PT), presented in one of two diametrically opposite positions. The authors of this study noticed that, irrespective of whether the animals’ head had been restrained, three neck muscles that would turn the head towards the target (obliquus capitis inferior, rectus capitis posterior maior and splenius capitis) exhibited a first transient burst of muscular activity ~90 ms after PT appearance, and a second period of tonic muscular activity lasting until the saccade onset (Fig 2b). Notably, the latency of the first response was too short to be explained by a voluntary motor command and, most importantly, it was time-locked to stimulus presentation, and not to the ensuing saccade (which could be performed up to 150 ms following the first transient burst, and whose latency had a remarkably higher variability compared to that of the first burst). Suggesting a functional significance of this phenomenon, the magnitude of the transient burst predicted the latency of the following saccade, as if the neck musculature was ‘warming up’ while the decision to move was being formed (16, 17).
The SLR pattern of EMG activity comprising two consecutive responses, a transient burst followed by a more sustained enhancement (Fig 2b), is strongly reminiscent of the CMR, which also entails two consecutive force increases, the first being more transient and the second being more tonic (see section [2]; Fig 1b). Bearing in mind that CMR and SLR studies entailed different measures, stimulus modalities, tasks and species, the reader might wonder whether it is justified to suggest a relationship between the SLR and the CMR. It is difficult to answer this question, especially considering that the paradigm used in the first SLR investigation entailed a voluntary movement overlapping with the late parts of the CMR (13). However, in a following study, the same group used a cueing task (18). Briefly, monkeys were trained to saccade to a target, but before the target appearance a task-irrelevant cue was presented at either the same or the opposite location of the following target. When cue and target were separated by a sufficiently long time (i.e. 600 ms), it appeared that the cue alone evoked the same two EMG modulations in the head-turning neck muscle (obliquus capitis inferior) (Fig. 2c). Thus, even in the absence of a subsequent overt action, the cue evoked the typical multi-phasic SLR pattern, making its similarity with the CMR striking. We believe that this similarity is worth being explored in the future (BOX 2).
BOX 2. Outstanding Questions.
Having highlighted a number of features shared across all RABs, we outline some research directions that we consider important to identify a putative shared neural mechanism.
Latencies. All reviewed RABs entail a transient modulation of muscular activity at ~100 ms following the stimulus (Fig. 5B). Does this similarity speak in favour of a shared neural mechanism? To address this question appropriately, future research will need to control for the different physiological parameters that are known to impact upon the latency of a muscular response (BOX 1). Should different RABs be subserved by a shared neural mechanism, then controlling for these sources of variability will permit to observe quasi-identical response latencies in each of them.
Multiple phases. CMR, SLR and OMC are associated with muscular responses entailing multiple phases. Instead, the two studies characterizing EMG during AS have described only one (inhibitory) phase (50, 51). Yet, it should be noted that recent work on rodents has suggested that AS might be achieved by the combination of two consecutive neural inhibitory processes: an early one only pausing the action, and a later one potentially cancelling it (102, 103). Future work should investigate the hypothesis of a multi-phasic AS process examining humans’ muscular activity, particularly considering the interesting analogies between AS and other RABs (104).
Supramodality. CMR, OMC and AS can be elicited by stimuli belonging to more than one sensory modality. Whether this is also the case in SLR (so far elicited only by visual stimuli) has never been explicitly tested, although a recent study showed that by pairing the SLR-evoking visual stimulus with somatosensory or auditory stimuli facilitates the SLR (105). Demonstrating that SLRs can in fact be evoked by non-visual stimuli would provide additional evidence in favour of a shared neural mechanism responsible for all RABs.
Active cortico-spinal drive. All RABs are typically or preferentially observed when the cortico-spinal drive is tonically active, i.e. while a participant executes an action or exerts a constant isometric force. While this feature can potentially link all RABs within a unifying neural mechanism, demonstrating that only some (but not all) RABs could be evoked in the absence of an active cortico-spinal drive would instead speak against such hypothesis.
Relationship with EEG signals. CMR and AS, when recorded simultaneously to the EEG, reveal to be tightly coupled to both event related potentials (5, 69) and neural oscillations (6, 106). Demonstrating a similar coupling in SLR or OMC would provide additional evidence in favour of a shared neural mechanism.
Inter-individual variability. A promising avenue of research pointing towards a shared neural mechanism could be comparing inter-individual variability in response magnitude across different RABs. Should the same neural mechanism be responsible for all RABs, then inter-individual variability should be similar across RABs (notably, this approach would require careful control of several parameters such as e.g. stimulus saliency or activity of the corticospinal drive).
These SLRs (observable even before a overt action) were interpreted as suggestive of a “reflexive covert orienting” mechanism useful to “warm up” the neck musculature while the possible decision to presumably move the head and the eyes in synergy is formed (16, 17). This functional interpretation is not different from that provided for the CMR. However, when it comes to hypothesize the neural circuits underlying these responses, these interpretations differ considerably, at least on the surface. In fact, SLRs have been mostly interpreted as the result of a largely subcortical machinery, involving the tecto-reticulospinal pathway and the superior colliculus (17, 19). Instead, the CMR – as the name itself implies – appears to be related to activity of the cerebral cortex, and specifically the activity (or the modulation) of the motor cortex. These two accounts are, however, not mutually exclusive. Indeed, it has recently been suggested that the cortex might contribute to the early SLR, for instance by priming the putative subcortical circuit with information related to higher-level processing of the sensory input or contextual and task specific constrains (20–22). Hence, it is conceivable that SLR and CMR might be unified as being subserved by a single neural mechanism or network – a hypothesis obviously requiring careful scrutiny.
[2.2]. SLRs in humans’ shoulder and arm muscles during reaching tasks
Following the first description in non-human primates, a number of studies have reported the existence of SLRs in humans (12, 23–26). Such studies have mostly adopted arm reaching tasks, often performed in the presence of a constant force field opposing the reach direction (27), given that a sustained background EMG activity appears to enhance the detectability of SLR (23). Again, the enhancement of the SLR in the presence of a stronger background force is reminiscent of the CMR, which is also optimally elicited during active isometric force exertion (5) and whose magnitude increases with enhanced background EMG activity (unpublished observation).
In the first SLR study in humans (12), participants could move their arm under a screen, while only the visual feedback of the hand position was provided as a coloured dot (Fig. 2d). Like in primate studies, participants had to hold the dot in a central position, and then move it to a peripheral target appearing in one among several possible locations. Intramuscular EMG was recorded from a number of shoulder (deltoid posterior, pectoralis major) and arm (triceps lateral, brachioradialis) muscles. The appearance of the peripheral target elicited a transient burst of muscle activity after approximately 100 ms (Fig. 2e). This initial burst of EMG activity was followed by the EMG activity consequent to the voluntary movement. Similarly to the SLR in primates, these EMG activities: (i) were spatially tuned, providing a glimpse of the pattern of muscle activation that would characterize the following voluntary action, and (ii) their amplitude predicted the overt response time latency.
SLRs fall nicely within the definition of RAB: they are evoked by fast-appearing stimuli, entail short-latency responses, and favour adaptive behaviour such as the preparation of an upcoming action in a spatially-tuned manner. A recent study (25) also examined whether the spatial tuning of the SLRs depends on volition – another feature defining RAB. The colour of a visual cue informed participants whether to perform a “reaching” movement towards a subsequently presented peripheral target, or an “anti-reaching” movement away from it (see also (28, 29), task adapted from (30)). This elegant design neatly dissociates the effects of stimulus position and goal position, which are congruent during “reaches” and incongruent during “anti-reaches” (Fig. 2f). The authors made an important observation: SLRs occurred in muscles necessary to move towards the target, irrespective of whether participants had to perform the reaching or anti-reaching movement (although, in the latter case, SLRs were slightly attenuated). In other words, the SLR implied the specification of a force useful for reaching the target, even though participants later voluntarily moved the arm to a diametrically opposite location. Thus, SLR depends mostly on the position of the suddenly-appearing visual target and can be only mildly modulated by volition.
[iii]. Online Motor Correction (OMC)
There is a third body of work investigating motor responses falling within the criteria that define RAB. This literature describes how sensory events cause adjustments during action execution. For example, while moving the arm to reach a cup, an unexpected event such as someone hitting your arm requires the movement to be corrected on the basis of proprioceptive and visual feedback. These adjustments are labelled Online Motor Correction (OMC). They are normally studied combining kinematic and EMG measures, and are assumed to be mediated by a number of cortical regions within the broad frontoparietal circuits that are often associated with goal-directed behaviour (3, 31–33). Like CMR and SLR, also OMC responses fulfil the criteria defining RAB.
The first studies of the neurophysiological processes underlying OMC date back to the middle of the last century. In the seminal work of Peter Hammond [(34, 35), reviewed in (36)], human subjects were required to exert a constant force to hold a weight attached to their wrist with a cable. A sudden perturbation – the pulling of the cable causing a stretch of the biceps muscle as well as displacement of the arm – evoked two EMG responses in the ipsilateral biceps: A first, short-latency monosynaptic response, peaking around 30 ms post stimulus (most likely reflecting a stretch reflex (37)), followed by a second polysynaptic response, occurring at 50-100 ms post stimulus. Notably, the second response was observed irrespectively of whether participants were instructed to “resist” or to “let go” the perturbation. This suggests that, like the previously reviewed CMR and SLR, this second polysynaptic response underling OMC is also weakly modulated by volition (but see more direct evidence below).
Later studies considerably enriched our understanding of the second polysynaptic response described by Hammond. We now know that this was likely the summation of (at least) two independent responses called R2 and R3, peaking ~60 and ~90ms post perturbation, and reflecting two distinct phases of a hierarchical OMC process (3, 38). Specifically, it has been proposed that R2 mostly reflects correction of “how” to achieve a given goal, such as which trajectory employing while reaching for a cup of coffee. Instead, R3 would mostly reflect correction of “what” goal to achieve (e.g. reaching for a cup of coffee vs. a different object nearby) (3, 39, 40).
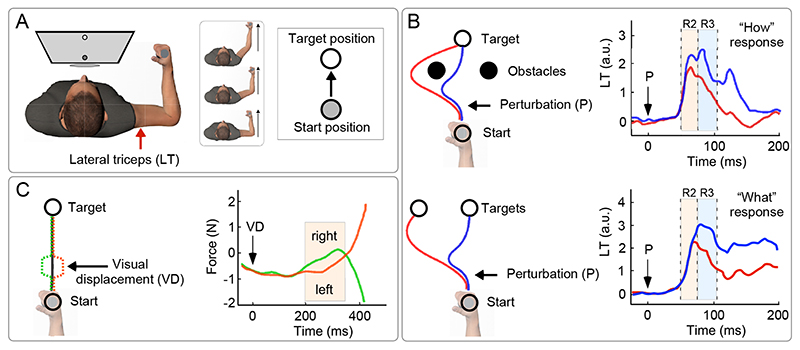
One elegant example of such functional dissociation was provided by Nashed et al. (39). Participants were instructed to reach a target with their arm (on a bi-dimensional plane) while a force was applied to activate their elbow extensor (lateral triceps), whose activity was recorded using EMG (Fig. 3a). Notably, participants were instructed to perform the task while avoiding two obstacles placed in between the start and the end position (Fig. 3b, top). On some trials, participants received a somatosensory perturbation that displaced their arm so that it would be likely to hit one of the two obstacles – hence requiring an OMC. It was observed that such OMC was adaptive, i.e. it was different depending on how far the perturbation had displaced the arm from the original pathway (note that the size of the arm displacement caused by the perturbation depended on small deviations in the arm trajectory prior to the perturbation, and not on the magnitude of the perturbation). In particular, following a large displacement of the arm, participants revised their pathway to circumvent the obstacles, while following a small displacement of the arm they stuck to the original pathway and reached the goal by passing between the obstacles. Such “optimized” correction – adaptively minimizing path length in a context-dependent manner – was underlined by a muscular burst observable ~60ms following the perturbation, i.e. during the R2 epoch (Fig. 3b, top).
Figure 3. Online Motor Correction (OMC).
(A) Participants are instructed to make an arm movement to reach a target with their hand (on a bi-dimensional plane) while a force was applied to activate their elbow extensor (lateral triceps, LT), which was recorded using EMG. Either a mechanical perturbation (P; panel b) or a visual displacement (VD; panel c) of the hand is used to trigger an OMC of the ongoing arm trajectory. (B) Participants receive a mechanical perturbation displacing their hand (black arrow). Top: If the correction implies a change of route in order to reach the goal (‘how’ change), an R2 is observed in LT ~60 ms post-perturbation. Bottom: If the correction implies a change of target to be reached (‘what’ change; note that after the perturbation the subject is allowed to choose whether hitting target A or B), an R3 is observed ~90 ms post-perturbation (displayed signals are obtained after subtracting the activity of unperturbed trials). Adapted from (39). (C) If participants are instructed to perform a voluntary movement in the same direction of a visual perturbation (VD in the right graph indicates the displacement onset at time = 0), an OMC identical to that observed without instruction (i.e. in the direction opposite to the perturbation) is observed, followed by the voluntary response in the same direction of the perturbation. Adapted from (43).
A complementary experiment tested the effect of a perturbation that did not prompt participants to change “how” to reach the target, but “which” target to reach (Fig. 3b, bottom) (39). Specifically, following the arm displacement, participants could freely choose whether to reach for the original target or for another target placed nearby to where the arm had been displaced. In this case the OMC, implying a revision of the movement goal, occurred ~90 ms following the perturbation, i.e. during the R3 time window (Fig. 3b, bottom).
Similarly to the CMR, OMCs are also supra-modal: they occur not only following somatosensory perturbations but also in reaction to sudden changes in the visual environment (41, 42). Also in the visual domain it is possible to distinguish between corrections of “how” to achieve a given goal, and “what” goal to achieve. For instance, coming back to the previous example of a hand reaching for a cup, a sudden change of the perceived hand position (consequent to a surreptitiously altered visual feedback of the hand position) would imply a correction of how to achieve a goal, while a change of position of the cup would imply a correction of which goal to achieve (8, 43). Notably, these two distinct changes evoke OMC occurring after ~90 ms (R2: “how” correction) and ~110 ms (R3: “what” correction), respectively (8). Given the longer processing time of visual input compared to proprioceptive input (Box 1), this result is reminiscent of the previously discussed hierarchical organization of R2 and R3 elicited by somatosensory stimuli, and of their functional significance [(3), but see (44)].
Another RAB-like feature of OMC – aligning it with both CMR and SLR – is that it is weakly modulated by volition. Evidence comes from a study by Franklin and Wolpert where participants moved their arm, visualized as a cursor, from a start to an end target (43). The cursor position was suddenly displaced (1.5 cm, orthogonally to the reaching direction) from the current hand position, and then swiftly restored to the actual hand position (the cursor displacement lasted 230 ms in total, see Fig. 3c). An OMC was observed in the EMG (pectoralis major), at ~100 ms following the onset of the cursor displacement, and in the force, at ~150 ms (BOX 1). To determine whether this OMC was voluntary or not, participants were instructed to perform a voluntary movement in the same direction of the perturbation, i.e. opposite to the OMC. Remarkably, even in this condition, there was an OMC identical to that observed in the experiment without instruction (i.e. in the direction opposite to the perturbation), followed by the voluntary response in the same direction of the perturbation (Fig. 3c). Thus, this experiment elegantly demonstrated that sensory-driven OMC are not voluntarily generated, but are largely automatic responses, poorly modulated by volition1.
[iv]. Action Stopping (AS)
Another scientific community (and related literature) investigates RAB by examining the interruption of an ongoing action following a sudden stimulus, which is labelled Action Stopping (AS). This phenomenon is reminiscent of OMC in that it entails a change of ongoing motor behaviour. However, here the emphasis is placed on the interruption, not on the correction. For instance, if someone hits your arm while you are reaching for an object in the dark, you might correct the movement trajectory (as in OMC), or you might stop the reaching movement entirely (as in AS). Under the assumption that AS is meant to prevent a future error (following the example above, not reaching the object), AS is often considered an adaptive behaviour (45, 46).
In the laboratory, AS is normally studied using the Stop Signal Task (SST) (47, 48). In the classic version of the SST, a “go signal” instructs a participant to perform an action such as pressing a button. After the action has been initiated, in a minority of trials, a sudden “stop signal” instructs the participant to interrupt the ongoing action. Depending on the time interval between the go signal and the stop signal, the action might or might not be successfully stopped. This allows the estimation of the following parameters: (i) the probability of stopping as a function of go-stop time interval, (ii) the response time of “go trials” (i.e. trials without a stop signal), and (iii) the response time of unsuccessfully stopped trials (which, notably, exhibits faster RTs compared to go trials). This information is used to compute what authors in this field call “stop signal reaction time”: a value, ranging between 200 and 300 ms, indexing how long it takes to voluntarily cease an ongoing action (47, 49).
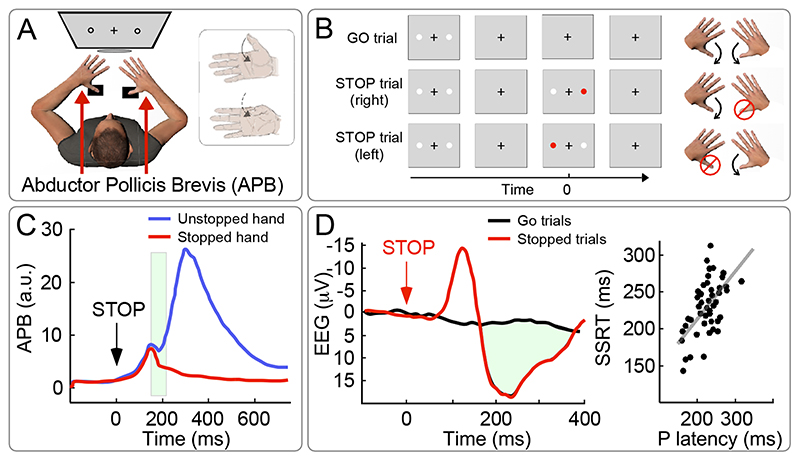
Surprisingly, nearly all studies using the SST have focused on estimating the stop signal reaction time, neglecting the modulation of the muscular activity before and during the actual stopping behaviour. Only a few recent studies have looked at this, using the following paradigm: on “go” trials, participants had to press two buttons in response to a visual cue, one with each hand. On “stop” trials, an additional (suddenly-appearing) visual stimulus prompted participants to suppress the response of one hand but to continue the response of the other hand [(50); Fig. 4A-B]. Surface EMG was measured from the abductor pollicis brevis of both hands, i.e. one of the muscles controlling the thumb, used for pressing the buttons. This paradigm allowed to sample EMG activity associated with both the stopped and the non-stopped action, and to compare those with the activity elicited by “go” trials. Two interesting observations were made. First, the EMG activity associated with successfully stopped actions displayed a short-latency inhibition, starting ~140 ms following the stop signal [(50); see also (51–53)]. This latency is compatible to (albeit slightly longer than2) the previously reviewed CMR, SLR and OMC. Second, a transient inhibition at the same latency was also present in the EMG measured from the other hand completing the task without stopping, implying that all ongoing actions were being stopped (Fig. 4C).
Figure 4. Action Stopping (AS).
(A-B) On “go” trials, in response to visual cues, participants have to press two buttons, one with each thumb. On “stop” trials, an additional sudden visual stimulus prompts participants to stop the movement of one thumb but to continue the movement of the other thumb. Surface EMG is measured from the abductor pollicis brevis (APB) of both hands, i.e. a muscle subserving the thumb response. (C) The “stop” signal evokes a short-latency inhibition in the APB associated with the interruption of the thumb movement, starting ~150 ms following the “stop” signal (green shaded area). Notably, a transient inhibition at the same latency is also observed in the APB of the thumb completing the task without stopping. Adapted from (50). (D) The sudden “stop” signal also evokes a negative-positive potential in the scalp EEG. The latency of the EEG positivity correlates (between participants) with the stop signal reaction time (SSRT). Adapted from (69).
The latter observation is very important when we consider that, at first glance, the SST appears qualitatively different from the tasks used for measuring CMR, SLR or OMC. In particular, SST entails a voluntary response to a stimulus, while all previously described RABs are largely automatic responses, i.e. scarcely modulated by volition. However, AS is not strictly driven by volition either, because all ongoing actions are stopped, not only those that are intended to be stopped. In other words, AS has a “global” character [as reviewed in (54)]. This observation is in line with the recent proposal that AS is not merely proactive, but also reactive to the surprising nature of the stop signal [(55, 56) see also (57)]. In line with this hypothesis, it is well known that slower response times or even non-voluntary stopping of ongoing actions can follow abrupt unexpected events, i.e. in a fully reactive mode. This has been shown in psychophysical studies testing unexpected events such as action errors, unexpected action outcomes, or unexpected perceptual events (54, 58–62), using distinct sensory modalities such as audition (63), vision (64) and somatosensation (65). Notably, some of the above classes of stimuli are extremely similar to those optimally eliciting the previously discussed RABs. For instance, OMC are elicited by stimuli that entail unexpected action outcomes or action errors, while the SLR and the CMR are elicited by unexpected perceptual events.
The neural origin of AS has mostly been explored using EEG. Here, another interesting similarity with the other described RAB emerges: Just like the stimuli inducing the CMR, also the “stop” stimuli discussed here evoke a widespread negative-positive potential (Fig. 4D). Moreover, and again in line with the CMR, the latency of the evoked positive wave robustly predicts the stop signal reaction time (50, 54, 66–68).
Concluding Remarks and Future Perspectives
The take-home message of this work is that a number of eye-opening similarities appear when the CMR, SLR, OMC and AS are critically compared. We have coined a unifying label for these phenomena – Reactive Adaptive Behaviour (RAB) – and defined four fundamental features that apply to all of them. These entail (i) the fast-rising nature of the RAB-evoking stimuli and, likewise, (ii) the fact that RAB occurs rapidly, within 150 ms following the stimuli. RAB is also (iii) adaptive, in that the behaviour is not stereotyped, but varies in response to the environmental context in a flexible manner that might ultimately enhance the efficiency of behaviour and, in the long term, survival. Finally, RAB is (iv) barely modulated by volition. A few additional similarities, albeit not yet conclusive, emerged. These are summarised in BOX 2, where we also suggest potentially fruitful pathways for future research.
These resemblances unavoidably trigger the question of whether all RABs have a common neural origin. Although we do not argue that all RABs rely on the very same neural structure, we do suggest that they likely share a common neural mechanism, perhaps working in synergy with RAB-specific cortical or subcortical structures. Such common mechanism is devoted to the rapid identification of important environmental events and the preparation of appropriate motor responses (10, 70–73). Note that several influential models of salience detection and orienting behaviour predict that salient events should have direct consequences on behaviour (74–77). Here we suggest that the RABs reviewed here (and possibly other similar behaviours) represent such consequences.
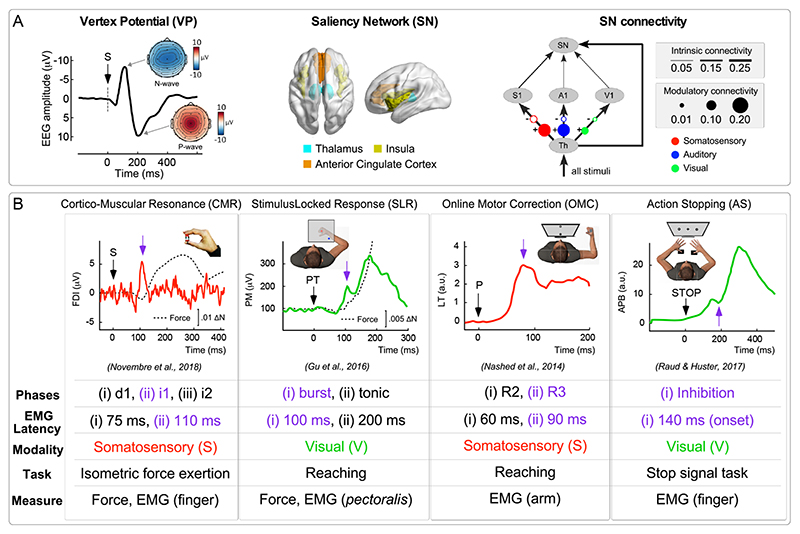
One particular neural system that could be responsible for RAB is the Salience Network (SN), comprising the insula, the anterior cingulate cortex, the thalamus, and a number of other subcortical structures (70, 75) (Fig. 5A). The SN is known to be activated by salient events through rapid pathways that bypass primary sensory cortices (72), in order to swiftly guide and adjust behaviour, for instance via the anterior cingulate cortex that facilitates rapid access to the motor system. Remarkably, the functional properties of the SN are reminiscent of those characterizing RAB. For instance, the electrocortical SN activity (in particular the activity of the insula and the anterior cingulate cortex (10, 78–80)) manifests itself as a transient negative-positive wave, maximal at the scalp vertex and therefore called vertex potential (VP) (9, 10) (Fig. 5A). Alike RAB, the VP also occurs swiftly after abrupt or unexpected sensory stimuli, and, crucially, irrespectively of their sensory modality (an important aspect to consider given that the reviewed RABs are similarly elicited by stimuli belonging to distinct sensory modalities) (9, 10). Furthermore, the VP magnitude is not stereotyped, but very sensitive to contextual changes in the sensory scene (71, 81–83), a feature compatible with RAB’s adaptive character. Finally, the VP is a very robust and largely automatic response, poorly modulated by volition. For instance, the VP is elicited by salient stimuli also in unconscious individuals, e.g. during sleep (84), and its magnitude appears to be minimally affected by high-level cognitive expectations about the stimulus nature (81).
Figure 5.
(A) Neural correlates of the Saliency Network (SN). Middle: the SN comprises the thalamus, the insula, and the anterior and middle cingulate cortex. Left: The SN activity manifests itself as a transient negative-positive electrocortical wave, maximal at the scalp vertex and therefore called vertex potential (VP). Right: Functional connectivity between the thalamus, the primary sensory cortices, and the cortical components of the SN (insular and cingulate cortex). The thickness of black lines line represents the strength of intrinsic connectivity. The size of colored dots/circles represents the strength of the modulatory effect exerted by external stimuli on each connection (colors represent stimulus modalities, plus (+) and minus (-) symbols represent enhancement and inhibition, respectively). Adapted from (72).(B) Illustration of all reviewed RABs, as characterized across four distinct studies (citations are embedded in the figure). Despite being elicited using clearly different tasks, all RABs entail an early, transient modulation of muscular activity at ~100 ms post-stimulus (purple arrow): the i1 of the CMR, the early burst of the SLRs, the R3 of the OMC and the inhibition of the AS. While we do not conclusively claim that these specific modulations are functionally equivalent, we stress that their slight differences in peak latencies can be explained by the sources of variability discussed in BOX 1. For instance, the longest latency of the AS response (discussed also in footnote 2) is consistent with the fact that it is evoked by visual stimuli (which are notoriously processed more slowly than somatosensory ones, see BOX 1) and measured in a distal muscle (the abductor pollicis brevis). Likewise, the shortest latency of the OMC response (in this example R3, but we could also consider R2) is compatible with the use of somatosensory stimuli eliciting it in a relatively proximal muscle (the lateral triceps).
The contribution of a cortical network such as the SN might at a first glance appear difficult to reconcile with the rapidity of RAB. The reader might wonder whether short-latency motor responses like RAB are too fast to be integrated with sensory information processed at cortical level. However, decades of work in both physiology and psychology has recognized the existence of fast pathways allowing the human brain to quickly process and react to sudden and unexpected sensory information (76, 85). Abrupt salient stimuli – such as the ones triggering RAB – can activate the SN very rapidly (86), through extralemniscal, non-modality-specific parallel thalamocortical connections that by-pass primary sensory regions (72, 87) (Fig. 5A). This comes at the cost of degrading the fidelity of stimulus coding and the resulting perceptual processing (76, 85). However, this rapidity permits the human brain to swiftly execute actions (31), in particular when certain sensory events call for urgent behaviour, with no need for fine-grained perceptual processing. Such a prioritised, extremely fast pathway appears to be a good candidate mediating RAB.
The cortical origin of RAB, and its putative relationship with the SN, should be investigated pairing behavioural or muscular recordings with simultaneous measurements of electrocortical activity. When this was done (e.g. using EEG in CMR and some AS studies), the effect of sensory stimulation was studied not only on muscular activity and kinematics, but also on brain activity, thereby leading to a more comprehensive characterization of how the nervous system responds to salient changes in the environment (5, 6, 66, 67). Notably, these studies show that the trial-by-trial variability in VP amplitude or latency predicts the trial-by-trial variability of the RAB of interest. This fruitful approach, once applied to the entire range of RABs, will establish their relationship with the cortical SN, and thereby identify a possible common mechanism. In fact, although a similar approach has not yet been attempted with SLR and OMC, there is indirect evidence for such common mechanism. For instance, an enhanced VP (i.e. increased in amplitude and decreased in latencies) is evoked by visual stimuli having strong visual contrast (higher luminance) (88), just like SLRs do (23). Along the same line, the well-known hierarchical organization of ERPs across time – with increasingly complex computations reflected in longer-latency components (89) – is reminiscent of the progressively more complex mechanisms underlying OMC responses: while early R2 might reflect “how” to achieve the given goal, the late R3 might reflect “what” goal to achieve (3).
Having highlighted the remarkable similarities characterizing the above-reviewed RABs, we suggested to unify these phenomena proposing a common neural mechanism related to the detection and reaction of salient environmental events. We wishfully expect this effort to trigger curiosity and cross-field fertilization.
Highlights.
Reactive Adaptive Behaviour (RAB) consists of short-latency motor responses to sudden sensory stimuli.
RAB is flexible and largely independent of volition.
We review four independent research fields, progressing in parallel and each characterizing a putatively distinct RAB.
We highlight remarkable similarities across the reviewed RABs.
We suggest that the reviewed RABs could be subserved by the same fundamental neural mechanism.
Acknowledgements
We wish to thank R. Caminiti, F. van Ede, R. Somervail, A. Fitzpatrick and two anonymous reviewers for useful comments on a previous version of this manuscript. GDI acknowledges the support of the Wellcome Trust (COLL JLARAXR) and of the European Research Council (Consolidator Grant PAINSTRAT).
Footnotes
The fact that OMC is poorly modulated by volition does not imply that this behaviour would not be consistent with what an individual would intentionally do in a given circumstance. Rather, it simply implies that this behaviour is triggered automatically by the stimulus without an individual deliberately choosing to act. Notably, according to some computational accounts such as the “optimal feedback control”, relatively complex goal-directed behaviours can be automatically produced following a sophisticated manipulation of sensory feedback (36, 107). From this perspective, OMC and voluntary actions could lead to the very same behaviour, being it subject to volition or not.
The latency of the EMG modulation associated with AS is relatively longer than the earliest EMG modulations associated with the RABs discussed so far (see Fig. 5). This apparent inconsistence might be explained by the fact that, in the SST, two distinct motor processes - the excitatory “go” and the inhibitory “stop” – compete with each other (Fig. 4B) (47). Importantly, because the “go” process is triggered before the “stop” process, the latter must override the former in order to successfully stop the ongoing action, which likely has a cost in latency. We have run preliminary simulations supporting this point.
References
- 1.Held R, Hein A. Movement-produced stimulation in the development of visually guided behavior. J Comp Physiol Psychol. 1963;56(5):872–876. doi: 10.1037/h0040546. [DOI] [PubMed] [Google Scholar]
- 2.Haggard P. Human volition: towards a neuroscience of will. Nat Rev Neurosci. 2008;9(12):934–46. doi: 10.1038/nrn2497. [DOI] [PubMed] [Google Scholar]
- 3.Scott SH. A Functional Taxonomy of Bottom-Up Sensory Feedback Processing for Motor Actions. Trends Neurosci. 2016;39(8):512–526. doi: 10.1016/j.tins.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Sechenov IM. Reflexes of the brain. MIT press; 1965. [Google Scholar]
- 5.Novembre G, et al. Saliency detection as a reactive process: unexpected sensory events evoke cortico-muscular coupling. J Neurosci. 2018;38(9):2474–17. doi: 10.1523/JNEUROSCI.2474-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novembre G, et al. The effect of salient stimuli on neural oscillations, isometric force, and their coupling. Neuroimage. 2019 Jan;198:221–230. doi: 10.1016/j.neuroimage.2019.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piitulainen H, et al. Phasic stabilization of motor output after auditory and visual distractors. Hum Brain Mapp. 2015;36(12):5168–5182. doi: 10.1002/hbm.23001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimitriou M, Wolpert DM, Franklin DW. The temporal evolution of feedback gains rapidly update to task demands. J Neurosci. 2013;33(26):10898–10909. doi: 10.1523/JNEUROSCI.5669-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bancaud J, Bloch V, Paillard J. Contribution EEG a letude des potentiels evoques chez lhomme au niveau du vertex. Rev Neurol (Paris) 1953;89(5):399–418. [PubMed] [Google Scholar]
- 10.Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol. 2009;101(6):3258–3269. doi: 10.1152/jn.91181.2008. [DOI] [PubMed] [Google Scholar]
- 11.Iannetti GD, Hughes NP, Lee MC, Mouraux A. Determinants of laser-evoked EEG responses: pain perception or stimulus saliency? J Neurophysiol. 2008;100(2):815–828. doi: 10.1152/jn.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pruszynski JA, et al. Stimulus-locked responses on human arm muscles reveal a rapid neural pathway linking visual input to arm motor output. Eur J Neurosci. 2010;32(6):1049–1057. doi: 10.1111/j.1460-9568.2010.07380.x. [DOI] [PubMed] [Google Scholar]
- 13.Corneil BD, Olivier E, Munoz DP. Visual responses on neck muscles reveal selective gating that prevents express saccades. Neuron. 2004;42(5):831–841. doi: 10.1016/s0896-6273(04)00267-3. [DOI] [PubMed] [Google Scholar]
- 14.Goonetilleke SC, et al. Cross-species comparison of anticipatory and stimulus-driven neck muscle activity well before saccadic gaze shifts in humans and nonhuman primates. J Neurophysiol. 2015;114(2):902–913. doi: 10.1152/jn.00230.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saslow MG. Effects of components of displacement-step stimuli upon latency for saccadic eye movement. J Opt Soc Am. 1967;57(8):1024–1029. doi: 10.1364/josa.57.001024. [DOI] [PubMed] [Google Scholar]
- 16.Corneil BD, Munoz DP, Chapman BB, Admans T, Cushing SL. Neuromuscular consequences of reflexive covert orienting. Nat Neurosci. 2008;11(1):13–15. doi: 10.1038/nn2023. [DOI] [PubMed] [Google Scholar]
- 17.Boehnke SE, Munoz DP. On the importance of the transient visual response in the superior colliculus. Curr Opin Neurobiol. 2008;18(6):544–551. doi: 10.1016/j.conb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- 19.Corneil BD, Munoz DP. Overt responses during covert orienting. Neuron. 2014;82(6):1230–1243. doi: 10.1016/j.neuron.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 20.Kozak RA, Corneil BD. High contrast, moving targets in an emerging target paradigm promote fast visuomotor responses during visually guided reaching. bioRxiv. 2021 doi: 10.1101/2021.01.27.428509. [DOI] [PubMed] [Google Scholar]
- 21.Contemori S, Loeb GE, Corneil BD, Wallis G, Carroll TJ. Trial-by-trial modulation of express visuomotor responses induced by symbolic or barely detectable cues. BioRxiv. 2021 doi: 10.1101/2021.01.29.428908. [DOI] [PubMed] [Google Scholar]
- 22.Contemori S, Loeb GE, Corneil BD, Wallis G, Carroll TJ. The influence of temporal predictability on express visuomotor responses. J Neurophysiol. 2021;125(3):731–747. doi: 10.1152/jn.00521.2020. [DOI] [PubMed] [Google Scholar]
- 23.Wood DK, Gu C, Corneil BD, Gribble PL, Goodale MA. Transient visual responses reset the phase of low-frequency oscillations in the skeletomotor periphery. Eur J Neurosci. 2015;42(3):1919–1932. doi: 10.1111/ejn.12976. [DOI] [PubMed] [Google Scholar]
- 24.Kozak RA, Kreyenmeier P, Gu C, Johnston K, Corneil BD. Stimulus-Locked Responses on Human Upper Limb Muscles and Corrective Reaches Are Preferentially Evoked by Low Spatial Frequencies. Eneuro. 2019;6(5):ENEURO.0301-19.2019. doi: 10.1523/ENEURO.0301-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu C, Wood DK, Gribble PL, Corneil BD. A Trial-by-Trial Window into Sensorimotor Transformations in the Human Motor Periphery. J Neurosci. 2016;36(31):8273–8282. doi: 10.1523/JNEUROSCI.0899-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu C, Pruszynski JA, Gribble PL, Corneil BD. A rapid visuomotor response on the human upper limb is selectively influenced by implicit motor learning. J Neurophysiol. 2019;121(1):85–95. doi: 10.1152/jn.00720.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott SH. Apparatus for measuring and perturbing shoulder and elbow joint positions and torques during reaching. J Neurosci Methods. 1999;89(2):119–127. doi: 10.1016/s0165-0270(99)00053-9. [DOI] [PubMed] [Google Scholar]
- 28.Chapman BB, Corneil BD. Neuromuscular recruitment related to stimulus presentation and task instruction during the anti-saccade task. Eur J Neurosci. 2011;33(2):349–360. doi: 10.1111/j.1460-9568.2010.07496.x. [DOI] [PubMed] [Google Scholar]
- 29.Munoz DP, Everling S. Look away: The anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5(3):218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- 30.Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18(10):1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- 31.Omrani M, Murnaghan CD, Pruszynski JA, Scott SH. Distributed taskspecific processing of somatosensory feedback for voluntary motor control. Elife. 2016 Apr;5:1–17. doi: 10.7554/eLife.13141. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Battaglia-Mayer A, et al. Impairment of online control of hand and eye movements in a monkey model of optic ataxia. Cereb Cortex. 2013;23(11):2644–2656. doi: 10.1093/cercor/bhs250. [DOI] [PubMed] [Google Scholar]
- 33.Battaglia-Mayer A, et al. Correction and suppression of reaching movements in the cerebral cortex: Physiological and neuropsychological aspects. Neurosci Biobehav Rev. 2014;42:232–251. doi: 10.1016/j.neubiorev.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Hammond PH. Involuntary activity in biceps following the sudden application of velocity to the abducted forearm. J Physiol. 1955;127(2):23–5P. [PMC free article] [PubMed] [Google Scholar]
- 35.Hammond PH. The influence of prior instruction to the subject on an apparently involuntary neuro-muscular response. J Physiol. 1956;132(1):17–8P. [PubMed] [Google Scholar]
- 36.Pruszynski JA, Scott SH. Optimal feedback control and the long-latency stretch response. Exp Brain Res. 2012;218(3):341–359. doi: 10.1007/s00221-012-3041-8. [DOI] [PubMed] [Google Scholar]
- 37.G T Liddell BE, Charles Sherrington S. Reflexes in Response to stretch. 1924:212–242. [Google Scholar]
- 38.Lee RG, Tatton WG. Motor Responses to Sudden Limb Displacements in Primates with Specific CNS Lesions and in Human Patients with Motor System Disorders. Can J Neurol Sci / J Can des Sci Neurol. 1975;2(3):285–293. doi: 10.1017/s0317167100020382. [DOI] [PubMed] [Google Scholar]
- 39.Nashed JY, Crevecoeur F, Scott SH. Rapid online selection between multiple motor plans. J Neurosci. 2014;34(5):1769–1780. doi: 10.1523/JNEUROSCI.3063-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cluff T, Scott SH. Apparent and actual trajectory control depend on the behavioral context in upper limb motor tasks. J Neurosci. 2015;35(36):12465–12476. doi: 10.1523/JNEUROSCI.0902-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saunders JA, Knill DC. Humans use continuous visual feedback from the hand to control fast reaching movements. Exp Brain Res. 2003;152(3):341–352. doi: 10.1007/s00221-003-1525-2. [DOI] [PubMed] [Google Scholar]
- 42.Brenner E, Smeets JBJ. Fast corrections of movements with a computer mouse. Spat Vis. 2003;16(3–4):365–376. doi: 10.1163/156856803322467581. [DOI] [PubMed] [Google Scholar]
- 43.Franklin DW, Wolpert DM. Specificity of reflex adaptation for taskrelevant variability. J Neurosci. 2008;28(52):14165–14175. doi: 10.1523/JNEUROSCI.4406-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cross KP, Cluff T, Takei T, Scott SH. Visual feedback processing of the limb involves two distinct phases. J Neurosci. 2019;39(34):3112–18. doi: 10.1523/JNEUROSCI.3112-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danielmeier C, Ullsperger M. Post-error adjustments. Front Psychol. 2011 Sep;2:1–10. doi: 10.3389/fpsyg.2011.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ullsperger M, Danielmeier C, Jocham G. Neurophysiology of performance monitoring and adaptive behavior. Physiol Rev. 2014;94(1):35–79. doi: 10.1152/physrev.00041.2012. [DOI] [PubMed] [Google Scholar]
- 47.Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychol Rev. 1984;91(3):295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- 48.Lappin JS, Eriksen CW. Use of a delayed signal to stop a visual reaction-time response. J Exp Psychol. 1966;72(6):805–811. [Google Scholar]
- 49.Band GPH, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychol (Amst) 2003;112(2):105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- 50.Raud L, Huster RJ. The Temporal Dynamics of Response Inhibition and their Modulation by Cognitive Control. Brain Topogr. 2017;30(4):486–501. doi: 10.1007/s10548-017-0566-y. [DOI] [PubMed] [Google Scholar]
- 51.Raud L, Westerhausen R, Dooley N, Huster RJ. Differences in unity: The go/no-go and stop signal tasks rely on different mechanisms. Neuroimage. 2020 Jan;210 doi: 10.1016/j.neuroimage.2020.116582. [DOI] [PubMed] [Google Scholar]
- 52.Coxon JP, Stinear CM, Byblow WD. Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol. 2006;95(6):3371–3383. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- 53.MacDonald HJ, Coxon JP, Stinear CM, Byblow WD. The fall and rise of corticomotor excitability with cancellation and reinitiation of prepared action. J Neurophysiol. 2014;112(11):2707–2717. doi: 10.1152/jn.00366.2014. [DOI] [PubMed] [Google Scholar]
- 54.Wessel JR, Aron AR. On the globality of motor suppression : unexpected events and their influence on behavior and cognition. Neuron. 2017;93(2):259–280. doi: 10.1016/j.neuron.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aron AR. From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69(12):e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wessel JR. Surprise: A More Realistic Framework for Studying Action Stopping? Trends Cogn Sci. 2018;22(9):741–744. doi: 10.1016/j.tics.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Braver TS. The variable nature of cognitive control: A dual-mechanisms framework Shifting the emphasis to variability in cognitive control. Trends Cogn Sci. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rabbitt PMA. Error Correction Time without External Error Signals. Nature. 1966;212(5060):1966. doi: 10.1038/212438a0. [DOI] [PubMed] [Google Scholar]
- 59.Logan GD, Crump MJC. Cognitive Illusions of Authorship Reveal Hierarchical Error Detection in Skilled Typists. Science (80-) 2010;330(6004):683–686. doi: 10.1126/science.1190483. [DOI] [PubMed] [Google Scholar]
- 60.Ruiz MH, Jabusch H-C, Altenmüller E. Detecting wrong notes in advance: neuronal correlates of error monitoring in pianists. Cereb Cortex. 2009;19(11):2625–39. doi: 10.1093/cercor/bhp021. [DOI] [PubMed] [Google Scholar]
- 61.Wessel JR, Danielmeier C, Morton JB, Ullsperger M. Surprise and Error: Common Neuronal Architecture for the Processing of Errors and Novelty. J Neurosci. 2012;32(22):7528–7537. doi: 10.1523/JNEUROSCI.6352-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gentsch A, Ullsperger P, Ullsperger M. Dissociable medial frontal negativities from a common monitoring system for self- and externally caused failure of goal achievement. Neuroimage. 2009;47(4):2023–2030. doi: 10.1016/j.neuroimage.2009.05.064. [DOI] [PubMed] [Google Scholar]
- 63.Schröger E. A Neural Mechanism for Involuntary Attention Shifts to Changes in Auditory Stimulation. J Cogn Neurosci. 1996;8(6):527–539. doi: 10.1162/jocn.1996.8.6.527. [DOI] [PubMed] [Google Scholar]
- 64.Berti S, Schröger E. Distraction effects in vision: behavioral and event-related potential indices. Neuroreport. 2004;15(4):665–669. doi: 10.1097/00001756-200403220-00018. [DOI] [PubMed] [Google Scholar]
- 65.Parmentier FBR, Ljungberg JK, Elsley JV, Lindkvist M. A Behavioral Study of Distraction by Vibrotactile Novelty. J Exp Psychol Hum Percept Perform. 2011;37(4):1134–1139. doi: 10.1037/a0021931. [DOI] [PubMed] [Google Scholar]
- 66.Wessel JR, Aron AR. Inhibitory motor control based on complex stopping goals relies on the same brain network as simple stopping. Neuroimage. 2014;103:225–234. doi: 10.1016/j.neuroimage.2014.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wessel JR, Huber DE. Frontal cortex tracks surprise separately for different sensory modalities but engages a common inhibitory control mechanism. PLOS Comput Biol. 2019;15(10):e1007420. doi: 10.1371/journal.pcbi.1006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kok A, Ramautar JR, De Ruiter MB, Band GPH, Ridderinkhof KR. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology. 2004;41(1):9–20. doi: 10.1046/j.1469-8986.2003.00127.x. [DOI] [PubMed] [Google Scholar]
- 69.Wessel JR, Aron AR. It’s not too late: The onset of the frontocentral P3 indexes successful response inhibition in the stop-signal paradigm. Psychophysiology. 2015;52(4):472–480. doi: 10.1111/psyp.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the “pain matrix.”. Neuroimage. 2011;54(3):2237–2249. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- 71.Moayedi M, et al. Laser-Evoked Vertex Potentials Predict Defensive Motor Actions. Cereb Cortex. 2015;25(12):4789–4798. doi: 10.1093/cercor/bhv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liang M, Mouraux A, Iannetti GD. Bypassing primary sensory cortices-a direct thalamocortical pathway for transmitting salient sensory information. Cereb Cortex. 2013;23(1):1–11. doi: 10.1093/cercor/bhr363. [DOI] [PubMed] [Google Scholar]
- 73.Gibson JJ. The ecological approach to visual perception. 1979. [Google Scholar]
- 74.Sokolov EN. Higher nervous functions: the orienting reflex. Annu Rev Physiol. 1963;25:545–580. doi: 10.1146/annurev.ph.25.030163.002553. [DOI] [PubMed] [Google Scholar]
- 75.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neumann O. Direct parameter specification and the concept of perception. Psychol Res. 1990;52(2–3):207–215. doi: 10.1007/BF00877529. [DOI] [PubMed] [Google Scholar]
- 77.Engbert R, Kliegl R. Microsaccades uncover the orientation of covert attention. Vision Res. 2003;43(9):1035–1045. doi: 10.1016/s0042-6989(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 78.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. Neurophysiol Clin. 2000;30(5):263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 79.Garcia-Larrea L, Frot M, Valeriani M. Brain generators of laser-evoked potentials: From dipoles to functional significance. Neurophysiol Clin. 2003;33(6):279–292. doi: 10.1016/j.neucli.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 80.Liberati G, et al. Nociceptive Local Field Potentials Recorded from the Human Insula Are Not Specific for Nociception. PLOS Biol. 2016;14(1):e1002345. doi: 10.1371/journal.pbio.1002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Valentini E, Torta DME, Mouraux A, Iannetti GD. Dishabituation of laser-evoked EEG responses: dissecting the effect of certain and uncertain changes in stimulus modality. J Cogn Neurosci. 2011;23(10):2822–2837. doi: 10.1162/jocn.2011.21609. [DOI] [PubMed] [Google Scholar]
- 82.Ronga I, Valentini E, Mouraux A, Iannetti GD. Novelty is not enough: laser-evoked potentials are determined by stimulus saliency, not absolute novelty. J Neurophysiol. 2013;44(3):692–701. doi: 10.1152/jn.00464.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moayedi M, et al. Nociceptive-Evoked Potentials Are Sensitive to Behaviorally Relevant Stimulus Displacements in Egocentric Coordinates. eNeuro. 2016;3(3):1–12. doi: 10.1523/ENEURO.0151-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bastuji H, Perchet C, Legrain V, Montes C, Garcia-Larrea L. Laser evoked responses to painful stimulation persist during sleep and predict subsequent arousals. Pain. 2008;137(3):589–599. doi: 10.1016/j.pain.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 85.Mountcastle VB, Poggio GF. In: Medical Physiology. CV Mosby Co, editor. CV Mosby Co; St. Louis: 1974. Structural organization and general physiology of thalamotelencephalic systems; pp. 227–253. [Google Scholar]
- 86.Ledberg A, Bressler SL, Ding M, Coppola R, Nakamura R. Large-scale visuomotor integration in the cerebral cortex. Cereb Cortex. 2007;17(1):44–62. doi: 10.1093/cercor/bhj123. [DOI] [PubMed] [Google Scholar]
- 87.Hu B. Functional organization of lemniscal and nonlemniscal auditory thalamus. Exp Brain Res. 2003;153(4):543–549. doi: 10.1007/s00221-003-1611-5. [DOI] [PubMed] [Google Scholar]
- 88.Hu L, Iannetti GD. Neural indicators of perceptual variability of pain across species. Proc Natl Acad Sci U S A. 2019 doi: 10.1073/pnas.1812499116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luck SJ. An Introduction to the Event-Related Potential Technique. MIT press; 2014. [Google Scholar]
- 90.Norman RW, Komi PV. Electromechanical delay in skeletal muscle under normal movement conditions. Acta Physiol Scand. 1979;106(3):241–248. doi: 10.1111/j.1748-1716.1979.tb06394.x. [DOI] [PubMed] [Google Scholar]
- 91.Corcos DM, Gottlieb GL, Latash ML, Almeida GL, Agarwal GC. Electromechanical delay: An experimental artifact. J Electromyogr Kinesiol. 1992;2(2):59–68. doi: 10.1016/1050-6411(92)90017-D. [DOI] [PubMed] [Google Scholar]
- 92.Jana S, Hannah R, Muralidharan V, Aron AR. Temporal cascade of frontal, motor and muscle processes underlying human action-stopping. Elife. 2020;9:1–28. doi: 10.7554/eLife.50371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Somervail R, et al. Waves of Change: Brain Sensitivity to Differential, not Absolute, Stimulus Intensity is Conserved Across Humans and Rats. Cereb Cortex. 2020 doi: 10.1093/cercor/bhaa267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iannetti GD, et al. Aδ nociceptor response to laser stimuli: Selective effect of stimulus duration on skin temperature, brain potentials and pain perception. Clin Neurophysiol. 2004;115(11):2629–2637. doi: 10.1016/j.clinph.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 95.Iannetti GD, et al. Evidence of a specific spinal pathway for the sense of warmth in humans. J Neurophysiol. 2003;89(1):562–570. doi: 10.1152/jn.00393.2002. [DOI] [PubMed] [Google Scholar]
- 96.Hu L, Cai MM, Xiao P, Luo F, Iannetti GD. Human brain responses to concomitant stimulation of Aδ and C nociceptors. J Neurosci. 2014;34(34):11439–11451. doi: 10.1523/JNEUROSCI.1355-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sollmann N, et al. The variability of motor evoked potential latencies in neurosurgical motor mapping by preoperative navigated transcranial magnetic stimulation. BMC Neurosci. 2017;18(1):1–15. doi: 10.1186/s12868-016-0321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ozaki I, Takada H, Shimamura H, Baba M, Matsunaga M. Central conduction in somatosensory evoked potentials: Comparison of ulnar and median data and evaluation of onset versus peak methods. Neurology. 1996;47(5):1299–1304. doi: 10.1212/wnl.47.5.1299. [DOI] [PubMed] [Google Scholar]
- 99.Tanosaki M, Ozaki I, Shimamura H, Baba M, Matsunaga M. Effects of aging on central conduction in somatosensory evoked potentials: Evaluation of onset versus peak methods. Clin Neurophysiol. 1999;110(12):2094–2103. doi: 10.1016/s1388-2457(99)00193-5. [DOI] [PubMed] [Google Scholar]
- 100.Sandhaeger F, von Nicolai C, Miller EK, Siegel M. Monkey EEG links neuronal color and motion information across species and scales. Elife. 2019;8:1–21. doi: 10.7554/eLife.45645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Frot M, Faillenot I, Mauguière F. Processing of nociceptive input from posterior to anterior insula in humans. Hum Brain Mapp. 2014;35(11):5486–5499. doi: 10.1002/hbm.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schmidt R, Leventhal DK, Mallet N, Chen F, Berke JD. Canceling actions involves a race between basal ganglia pathways. Nat Neurosci. 2013;16(8):1118–1124. doi: 10.1038/nn.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schmidt R, Berke JD. A pause-then-cancel model of stopping: Evidence from basal ganglia neurophysiology. Philos Trans R Soc B Biol Sci. 2017;372(1718) doi: 10.1098/rstb.2016.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tatz JR, Soh C, Wessel JR. Towards a two-stage model of action-stopping: Attentional capture explains motor inhibition during early stop-signal processing. bioRxiv. 2021 doi: 10.1101/2021.02.26.433098. [DOI] [Google Scholar]
- 105.Glover IS, Baker SN. Multimodal stimuli modulate rapid visual responses during reaching. J Neurophysiol. 2019;122(5):1894–1908. doi: 10.1152/jn.00158.2019. [DOI] [PubMed] [Google Scholar]
- 106.Swann N, et al. Intracranial EEG Reveals a Time- and Frequency-Specific Role for the Right Inferior Frontal Gyrus and Primary Motor Cortex in Stopping Initiated Responses. J Neurosci. 2009;29(40):12675–12685. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Todorov E, Jordan MI. Optimal feedback control as a theory of motor coordination. Nat Neurosci. 2002;5(11):1226–1235. doi: 10.1038/nn963. [DOI] [PubMed] [Google Scholar]