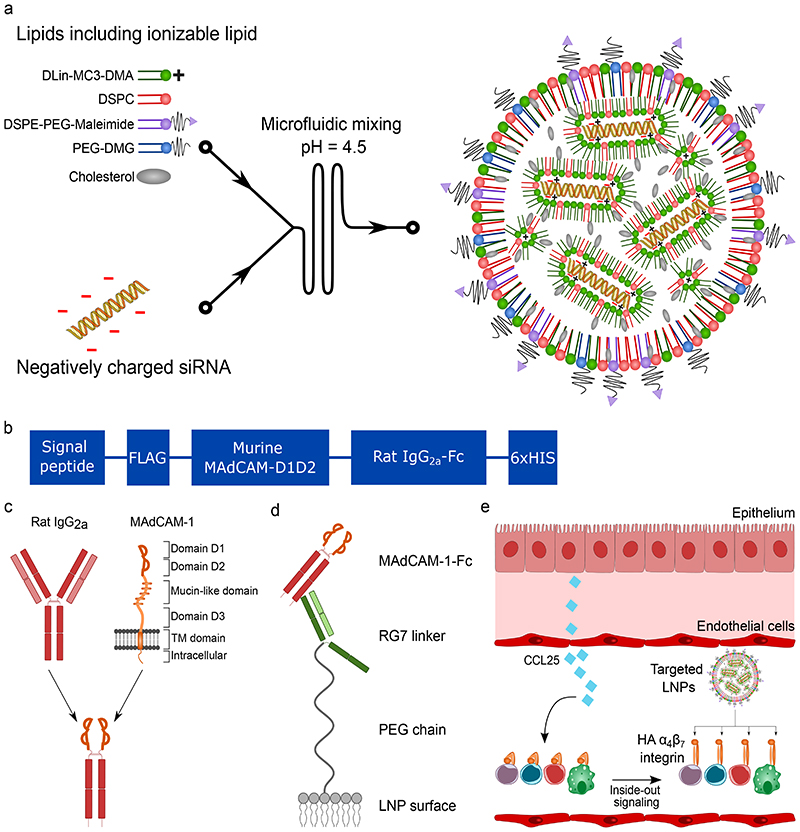
Figure 1. Generation of LNPs to target the high-affinity conformation of integrin α4β7.
a. Illustration of the generation of LNPs using microfluidics. The ionizable lipid facilitates siRNA encapsulation through its positive charge at low pH. b. Overview of the different domains of the MAdCAM-1-Fc fusion protein. c. Overview of the fusion strategy. The different domains of the wild type MAdCAM-1 are shown. Only the integrin binding domains D1 and D2 are used. D1D2 is fused to the hinge of rat IgG2a with a flexible linker. d. Schematic drawing depicting the conjugation strategy of the MAdCAM-1-Fc to the LNPs. The RG7 linker (mAb against rat IgG2a) is chemically conjugated with the LNPs to the maleimide group in the lipid DSPE-PEG-Maleimide. RG7 readily binds the MAdCAM-1-Fc by antibody affinity. e. LNP targeting to HA α4β7 integrin. CCL25 induces the integrin conformational change.