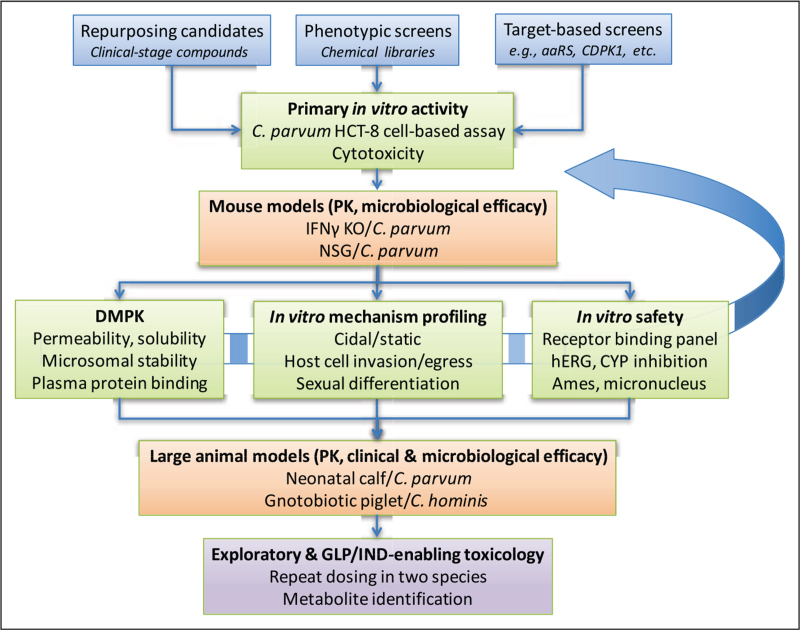
FIGURE 1.
Screening cascade for cryptosporidiosis therapeutic candidates. Starting compounds can be identified from collections of clinical-stage compounds, phenotypic screening of chemical libraries, or screens of previously characterized or rationally selected targets. Regardless of the source, compounds are first screened for activity in a standardized cell-based assay such as C. parvum infection of HCT-8 cells. Hits are counterscreened for cytotoxic effects and typically a 20-fold or higher margin is required for progression. The next step is characterizing both PK and microbiological efficacy as determined by reduction in oocyst shedding in a mouse model. Compounds with in-vivo activity are then characterized for several parameters in parallel, including DMPK properties, in-vitro safety assays, and further in-vitro profiling, such as for activity at specific stages of the parasite lifecycle. This may involve an iterative process of repeated medicinal chemistry optimization and retesting with in-vitro and in-vivo assays (curved arrow). Only compounds with confirmed drug-like properties are tested in expensive and limited-capacity large animal models for pharmacokinetics and clinical (reduction in diarrheal stool output) and microbiological efficacy. Only compounds with demonstrated large animal efficacy would be advanced to exploratory (non-GLP) toxicology studies to de-risk and then GLP toxicology to enable IND-filing and clinical testing. aaRS, aminoacyl-tRNA synthetase; CDPK1, calcium dependent protein kinase 1; CYP, cytochrome P450; DMPK, drug metabolism and pharmacokinetics; GLP, Good Laboratory Practices; hERG, human Ether-a-go-go-Related Gene; IFNγ KO, interferon gamma knockout; IND, Investigational New Drug application; NSG, NOD SCID gamma; PK, pharmacokinetics.