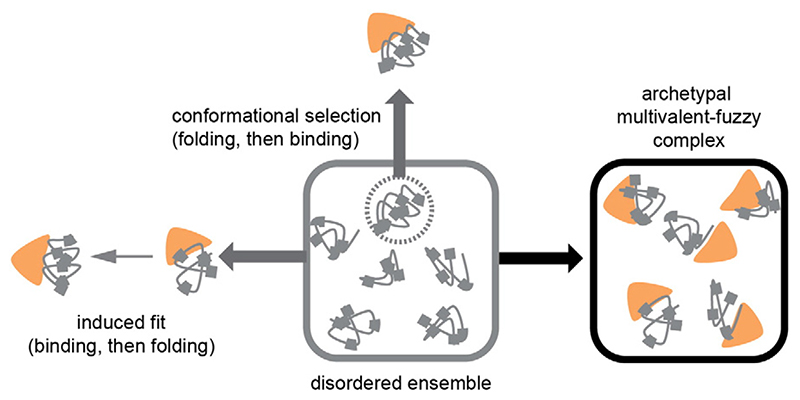
Fig. 3.
The different binding modes observed for IDP interactions (shown in gray, with valencies indicated by gray square markers) with folded proteins (orange). Due to their dynamics, IDPs populate a disordered ensemble in isolation [109]. In the “conformational selection” binding mode, the folded binding partner can bind to a specific conformation of the ensemble, which can also be a folded state. In the “induced-fit” binding mode, the presence of the binding partner induces folding of the IDP. For FG-domain-NTR interactions, it appears as if the native-state ensemble tends to bind to NTRs so that many conformations can readily engage with the NTR without requiring much time or energy. The result appears to be an archetypal, multivalent fuzzy complex that can form remarkably quickly (reprinted from ref. [46]).