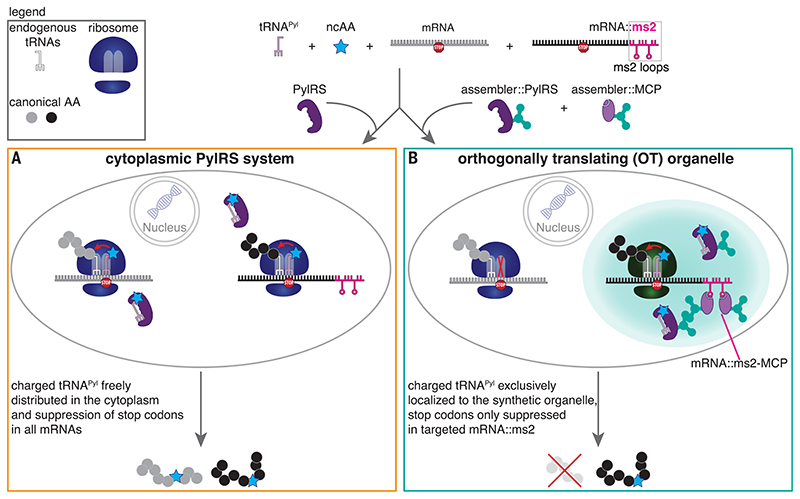
Fig. 1. Spatial separation of the necessary components to enable orthogonal translation to decode a specific stop codon in a specifically tagged mRNA.
(A) Expression of the synthetase PylRS leads to aminoacylation of its cognate stop codon suppressor tRNAPyl with a custom designed ncAA. This leads to site-specific ncAA incorporation whenever the respective stop codon occurs in the mRNA of the POI. Given that many endogenous mRNAs terminate on the same stop codon, using this approach in the cytoplasm potentially leads to misincorporation of the ncAA into unwanted proteins. (B) To avoid this, we propose to spatially enrich all components to an OT organelle, including the mRNA of the POI, the aminoacyl-tRNA synthetase, the tRNA, and ribosomes through the use of “assemblers.” Aminoacylated tRNAPyl should only be available in direct proximity of the OT organelle, so that only here stop codon suppression can occur. The corresponding stop codon in mRNAs that are not targeted to the OT organelle should not get translated. Whereas in (A) GCE is stop codon specific, in (B), it is stop codon– and mRNA-specific.