Fig. 3.
Proposed mechanisms for IL-6-sustained hypofunctional Treg state in psychosis.
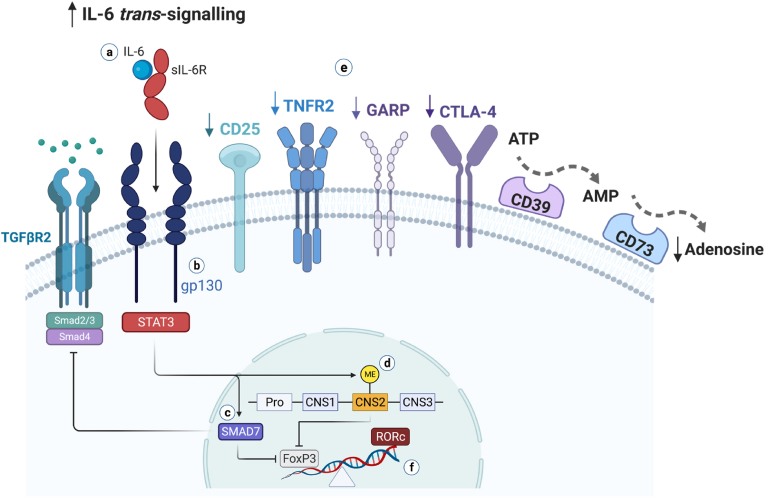
We summarise mechanisms through which IL-6 might interfere with the immunosuppressive function of Tregs in psychosis (for references, see the main text). a) The IL-6 pro-inflammatory pathway, named trans-signalling, is formed by IL-6 bound to its naturally occurring soluble receptor (sIL-6). The IL-6/sIL-6 complex associates with two molecules of the transmembrane signal transducer glycoprotein (gp130), activating the JAK/STAT3 pathway. b) It has been shown that subpopulations of human Tregs that are hypofunctional to suppress inflammation and related cellular mediators express more gp130; this renders more plasticity to Tregs, turning them more prone to FoxP3 destabilisation upon IL-6/sIL6R biding and conversion to pathogenic T cells under inflammatory conditions. c) IL-6 trans-signalling upregulates the expression of SMAD7, a negative regulator of TGF-β signalling, which inhibits the expression of FoxP3 and the generation of functional Foxp3+ Tregs. d) Complete demethylation of the conserved non-coding sequence 2 (CNS2), a CpG-rich island in the FoxP3 locus (also known as Treg cell-specific demethylated region), is crucial to Treg stability and suppressive function. IL-6-STAT3 induces CNS-2 methylation, resulting in FoxP3 destabilisation. e) Hypofunctional Tregs are deficient in expressing molecules that are implicated in their functional capacity, such as CD25, TNFR2, GARP, CTLA-4, and the adenosine-producing ectoenzymes CD39 and CD73 (see also Table 4 in the main text); adenosine is a potent anti-inflammatory molecule. The hypofunctional Treg state restrains the regulation of pro-inflammatory innate and adaptive forces. f) By inhibiting or destabilising FoxP3 expression, mechanisms c and d skew the T cell fate towards pathogenic T cells, triggering the expression of RORc, the Th17-transcriptional factor that is the polar opposite to FoxP3+ Treg differentiation. Such IL-6-induced FoxP3 destabilisation mechanisms are also likely relevant in naïve T cells, culminating in a self-sustaining state of mild peripheral immune activation with less generation of new competent Tregs. Figure adapted fromCorsi-Zuelli and Deakin (2021).