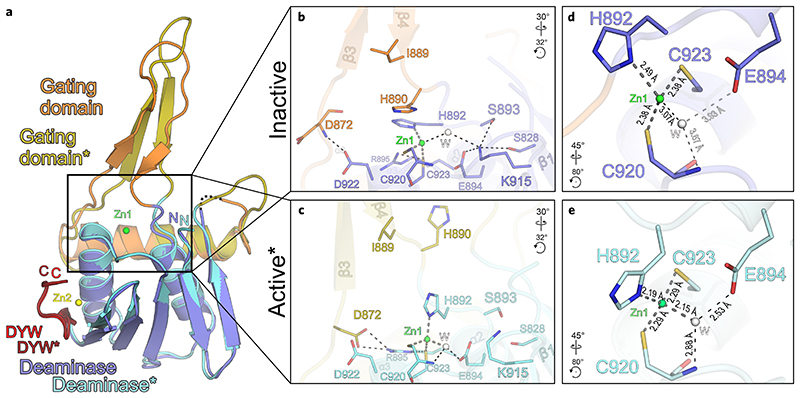
Fig. 3. The DYW gating domain regulates cytidine deamination catalysis.
a, Superimposition of inactive OTP86DYW (colouring and dashed lines are as in Fig. 1b) and activated OTP86DYW★ (deaminase domain, cyan; gating domain, ochre; DYW motif, dark red). Zn1 (green) and Zn2 (yellow) of OTP86DYW are shown. b, A close-up view of the OTP86DYW active site in the inhibited state. c, A close-up view of the activated OTP86DYW★ active site in a catalytically competent conformation. d, A close-up view of the Zn1-coordination environment of the OTP86DYW active site in its catalytically inhibited state. e, A close-up view of the Zn1-coordination environment of the OTP86DYW active site in its catalytically active state. Distances within the vicinity of Zn1 are given in Å. Rotation symbols indicate the views relative to a. Interacting residues are shown as sticks and coloured by atom type. Water molecules (W) shown as white spheres. Carbon — as for the respective molecule; nitrogen, blue; oxygen, red; sulfur, yellow. Dashed lines represent hydrogen bonds, thick grey dashed lines indicate zinc coordination.