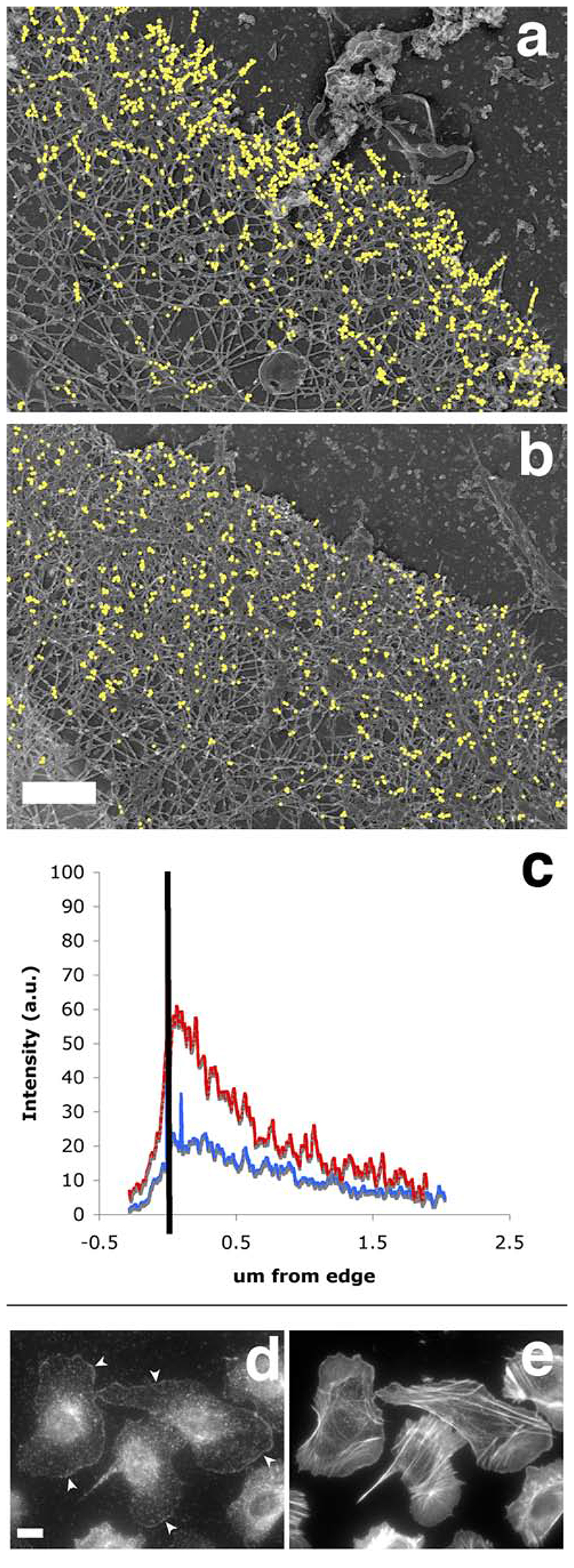
Figure 10.
The Arp2/3-mediated in situ polymerisation pattern is distinct from the free barbed end distribution, and closely matches the distribution of the cytoskeletal-bound fraction of the ar2/3 complex. A polymerisation mix containing actin (“actin only”, a, c) or actin plus Arp2/3 plus VCA (“actin + activated Arp2/3”, b, c) was incubated on extracted cytoskeleton for 1 min before the cells were fixed and processed for replica microscopy. Photos of the leading edges were taken at high resolution and these were processed to analyse the distribution of incorporated labelled actin as described in Material and Methods. a and b show the exogenous actin incorporation in representative leading edges from an “actin only “ sample and an “actin+ activated Arp2/3” sample respectively, where gold particles have been digitally enhanced for easier visualisation (bar, 0.5 um). c: average distribution of gold particles at the leading edge of the cells. Black bar represents the position of the leading edge, with the area on the left being outside the cell. Red, average distribution of exogenous actin in 6 “actin only “ samples; blue, average distribution of exogenous actin in 16 “actin+ activated Arp2/3” samples. d: the cytoskeleton–bound pool of Arp2/3 complex is mostly at the leading edge, mimicking the nucleation pattern in cells. Resting MTLn3 cells were extracted for 1 minute with 0.1% of Triton in cytoskeletal buffer containing 1 ug/ml phallacidin, and fixed and stained for F-actin using labelled phalloidin (right) and for Arp2/3 complex using anti-p34 antibodies (left). Detergent extraction of live cells eliminates most of the Arp2/3 complex in cytoplasm whilst a large portion of the Arp2/3 complex at the leading edge remains intact (arrowheads). Bar. 10 um.