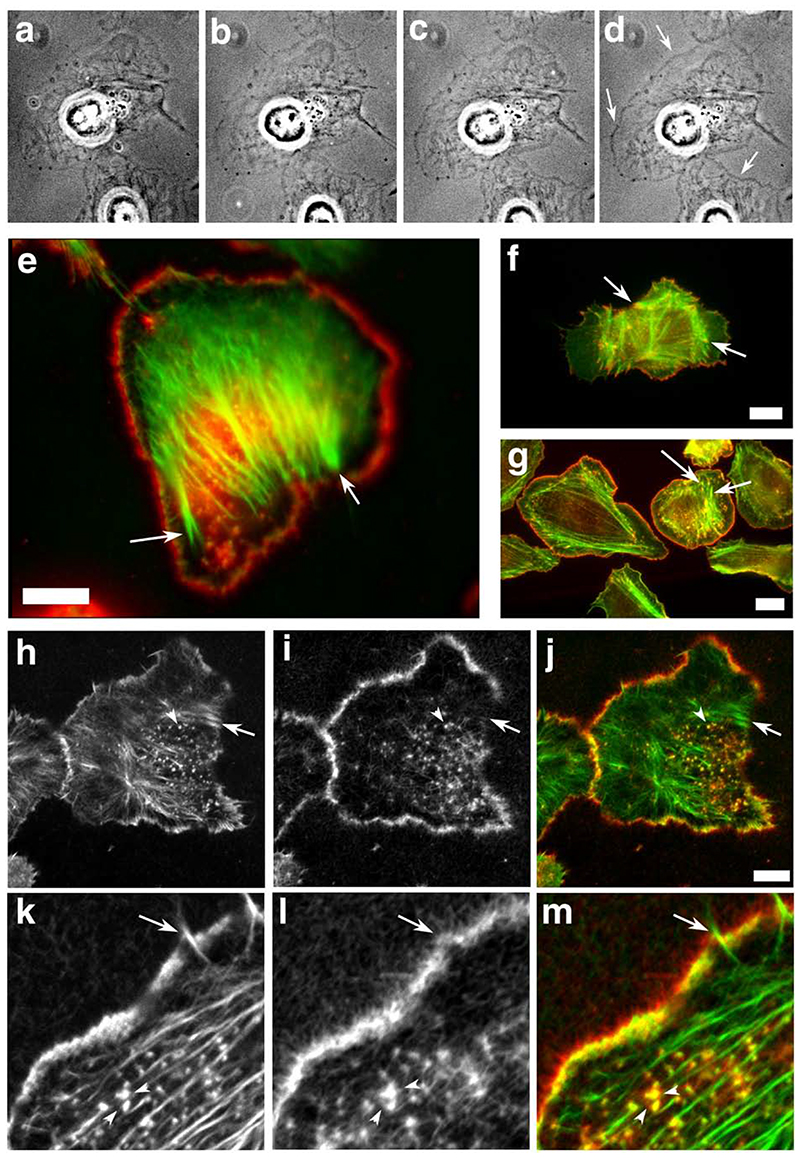
Figure 5.
Scar-WA-activated, -Arp2/3-mediated actin polymerisation in permeabilised resting cells using purified proteins results in a leading edge nucleation pattern similar to that of EGF-stimulated cells. Resting MTln3 cells were membrane-extracted and the resulting cytoskeletons were incubated with an Arp2/3 polymerisation mix (2 uM biotinylated actin, 5 nM Arp2/3, 20 nM Scar-WA, 0.2 uM cofilin) for 5-10 minutes. The samples were imaged live using fluorescence contrast (a-d), and/or fixed and processed for light (e) or confocal (h-m) microscopy. WA domain-activated Arp2/3 complex preferentially induces actin polymerisation at the leading edge in permeabilized cells in a pattern similar to barbed end generation after EGF stimulation. a-d: time course of the actin mix polymerisation in permeabilised cells as visualised by phase contrast microscopy. Actin accumulation around the cell circumference is visible as a denser grey outline of the cell (arrows). a, 40 sec after the beginning of membrane extraction, immediately before polymerisation mix addition; b-d, 1, 3 and 8 min after mix addition. e, h-m: analysis of the distribution of the de-novo Arp2/3 mediated network (green and h, k: pre-existing filaments as identified by phalloidin staining; red and i, l, newly polymerised network as identified using Cy3 coupled anti-biotin antibodies). k-m shows a detail of the leading edge of a cell after polymerisation of the mix. For comparison, light microscopy images of standard nucleation activity using monomeric actin polymerisation (standard barbed end distribution as in Figure 3; green, F-actin, red, newly polymerised actin) is shown for resting (f) and EGF-stimulated cells (g). Arrows point to focal contacts that show standard nucleation activity in both resting and stimulated cells, but are devoid of newly polymerised Arp2/3 mediated network. Arrowheads points at actin-rich dots inside the cell which show both standard actin nucleation and Arp2/3-mediated nucleation activity. Bars, 10 um.