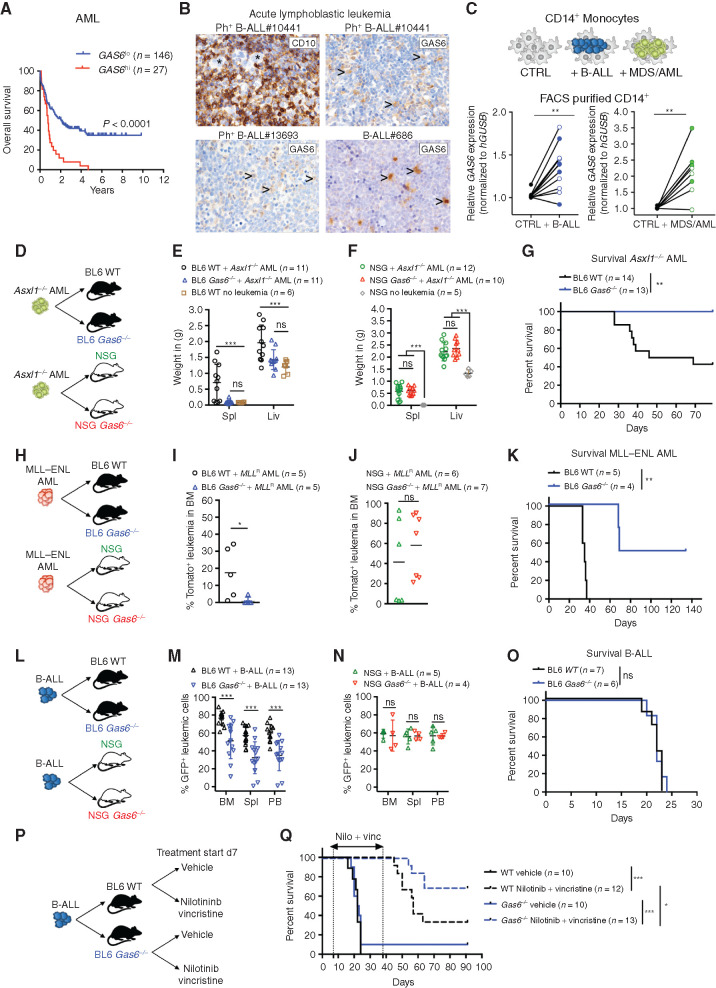
Figure 1.
Leukemia-induced GAS6 contributes to immune evasion and leukemic progression. A, Prognostic value of GAS6 expression in AML (TCGA LAML, n = 173). Data was generated using the KaplanScan mode from the R2 Genomics Analysis and Visualization Platform (http://r2.amc.nl). Survival analysis by log-rank (Mantel–Cox) test. B, IHC of GAS6 on bone marrow trephine biopsies from representative patients with Ph+ (BCR–ABL1+) B-ALL at diagnosis. CD10 marks B-ALL blasts (upper left). Arrowhead marks myeloid cells. C, CD14+ peripheral blood monocytes from healthy donors were cultured with leukemia cells from either patients with Ph+ B-ALL (left, CD14+ from 11 donors cultured with 2 Ph+ B-ALL) or patients with myeloid diseases (right, CD14+ from 8 donors cultured with 1 AML and 1 higher-risk MDS). GAS6 mRNA levels were then determined by real-time PCR. Each data point represents a mean value obtained from two technical replicates, after normalization to a reference gene, GUSB. **, P < 0.01, paired two-tailed Student t test. Characteristics of all patients and healthy donors are described in Supplementary Table S1. D–F, Wild-type (WT) and Gas6−/− C57BL/6 or NSG (NSG Gas6−/−, line#697-31) mice were challenged with Asxl1−/− leukemia cells (105). Weight of spleens and livers on day 19 days post–leukemia challenge are displayed. NSG (n = 5) and C57BL/6 (n = 6) mice without leukemia are used as reference. ns, not significant. ***, P < 0.001, paired two-tailed Student t test. G, Kaplan–Meier survival analysis of WT and Gas6−/− C57BL/6 challenged with Asxl1−/− leukemia cells (105). **, P < 0.01, log-rank (Mantel–Cox) test. H–J, WT and Gas6−/− C57BL/6 or NSG (NSG Gas6−/−, line#697-31) mice were challenged with MLL–ENL AML cells (105). BM aspiration was performed after 22 days to determine leukemic burden (Tomato+). ns, not significant. *, P < 0.05, paired two-tailed Student t test. K, Kaplan–Meier survival analysis of WT and Gas6−/− C57BL/6 challenged with MLL–ENL AML cells (105). **, P < 0.01, log-rank (Mantel–Cox) test. L–N, WT and Gas6−/− C57BL/6 or NSG (NSG Gas6−/−, line#697-29) mice were challenged with B-ALL cells (103) and analyzed two weeks post–leukemia injection for leukemic burden (B220+GFP+) in bone marrow (BM), spleen (Spl), and peripheral blood (PB). This experiment was repeated with a different NSG Gas6−/− mouse line (line#697-31) with similar results (Supplementary Fig. S3E). ns, not significant. ***, P < 0.001, paired two-tailed Student t test. O, Kaplan–Meier survival analysis of WT and Gas6−/− C57BL/6 challenged with B-ALL cells (103). ns, not significant, log-rank (Mantel–Cox) test. P and Q, Kaplan–Meier survival analysis of WT and Gas6−/− C57BL/6 challenged with B-ALL cells (103) and subjected to either vehicle or nilotinib plus vincristine treatment combination. Treatment was initiated on day 7 and terminated on day 39. Data representative of at least two independent experiments. *, P < 0.05; ***, P < 0.001, log-rank (Mantel–Cox) test.