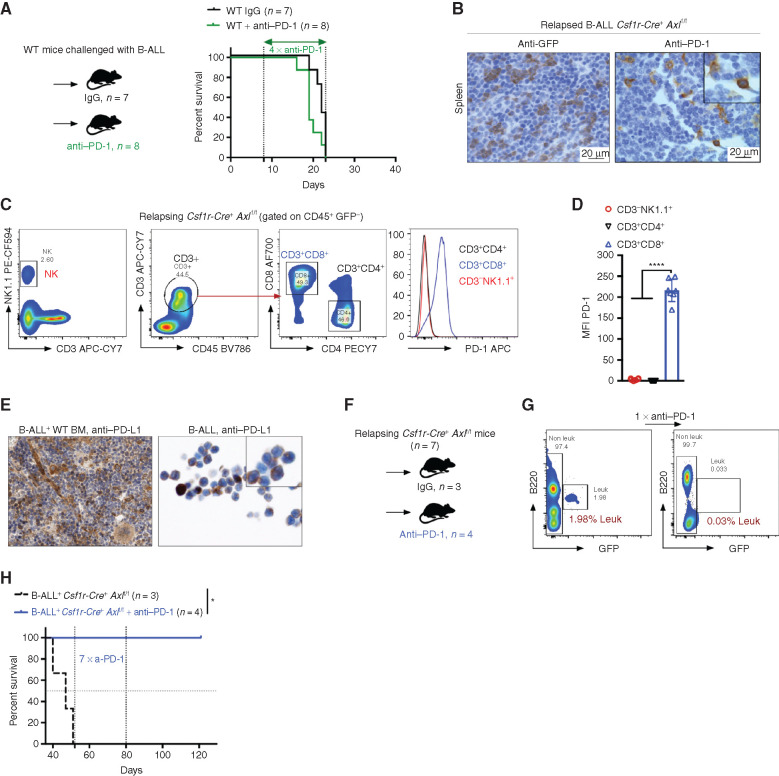
Figure 5.
Axl deficient macrophages trigger antileukemic immunity and elicit PD-1 checkpoint blockade. A, Kaplan–Meier survival analysis of WT mice challenged with 103 B-ALL cells and treated with either anti–PD-1 (n = 8) or isotype control (n = 7). B, GFP+ blasts (left) and PD-1+ cells (right) by IHC in the spleen of Csf1r-Cre+ Axlf/f mice that succumbed to B-ALL with a delayed latency of >40 days (Mice depicted in Fig. 2C). C and D,Csf1r-Cre+ Axlf/f from three independent experiments were followed by weekly bleeding to identify mice with late disease recurrence (n = 7). Flow cytometry data depicting PD-1 expression in peripheral blood lymphocytes (CD4 and CD8 T cells, NK cells) and corresponding PD-1 mean fluorescence intensity (MFI) from Csf1r-Cre+ Axlf/f mice showing signs of relapse (detectable GFP+ cells, representative data in G). ****, P < 0.0001, unpaired two-tailed Student t test. E, PD-1 ligand (PD-L1) expression by IHC in bone marrow cells with both stromal and hematopoietic morphology (left), as well as on cytospined B-ALL cells (right). F and G,Csf1r-Cre+ Axlf/f mice with late disease recurrence (n = 7, depicted in C and D) were either left untreated (n = 3) or subjected to 7 cycles of anti–PD-1 treatment (n = 4; 200 μg/mouse every 4 days). Representative FACS plot depicting leukemic burden (GFP+ B220dim) in the peripheral blood of the same mouse before and after one shot of anti–PD-1 treatment. H, Kaplan–Meier survival analysis of mice from F. *, P < 0.05, log-rank (Mantel–Cox) test.