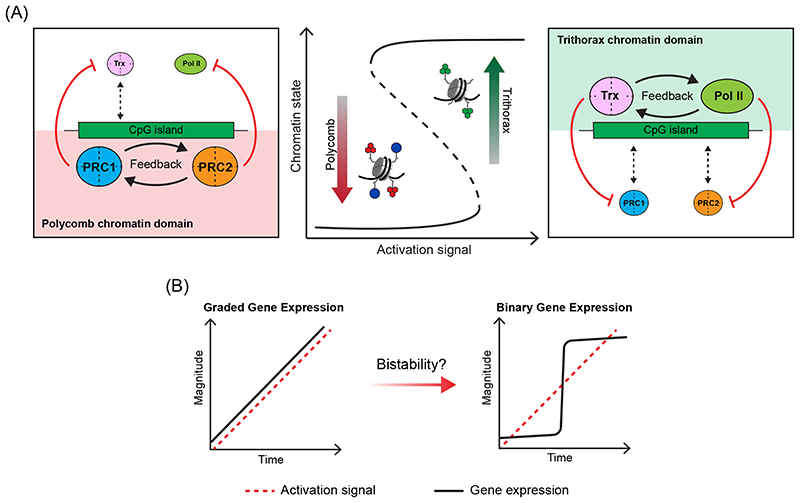
Figure 7. CpG islands and chromatin bistability.
(A) A schematic illustrating how chromatin bistablity could form at CpG islands (CGIs). When transcription activation signals are low or absent, communication and feedback between Polycomb repressive complex 1 (PRC1) and PRC2 could drive the formation of repressive Polycomb chromatin domains that antagonise Trithorax (Trx) complexes and RNA polymerase II (Pol II) activity (left). When activation signals are high and persistent, communication and feedback between Trithorax complexes and Pol II could drive the formation of transcription-permissive Trithorax chromatin domains that antagonise PRC1 and PRC2 (right). The capacity of both Polycomb and Trithorax systems to sample CGIs coupled with the feedback and antagonistic mechanisms inherent to the formation of each chromatin state, would provide the opportunity to switch between predominantly Polycomb or predominantly Trithorax chromatin states as gene activation signals increase or decrease. We speculate that this mode of gene regulation could help to shape gene expression transitions (see part B) and also provide a chromatin-encoded hysteresis of the current transcriptional state of the gene in the face of inherently stochastic and pulsatile transcription initiation induced from single gene promoters.
(B) If transitions between gene expression states, for example gene induction during cellular differentiation, scaled linearly with its activation signal, then one would predict graded expression output (left). However, if CGIs help to create bistable chromatin at gene promoters, this could shape binary, switch-like, gene expression transitions in which Polycomb chromatin domains constrain activation signals until appropriate activation thresholds are reached, at which point transcription initiation would precipitate a rapid switch into a Trithorax chromatin state and potentiate transcription. In the context of such a system, one might predict CGIs could help to convert graded gene activation signals into binary switch-like gene expression outputs through chromatin bistability. This could be particularly useful in supporting decisive gene expression transitions during development.
Adapted with permission from REF. 290, Elsevier.