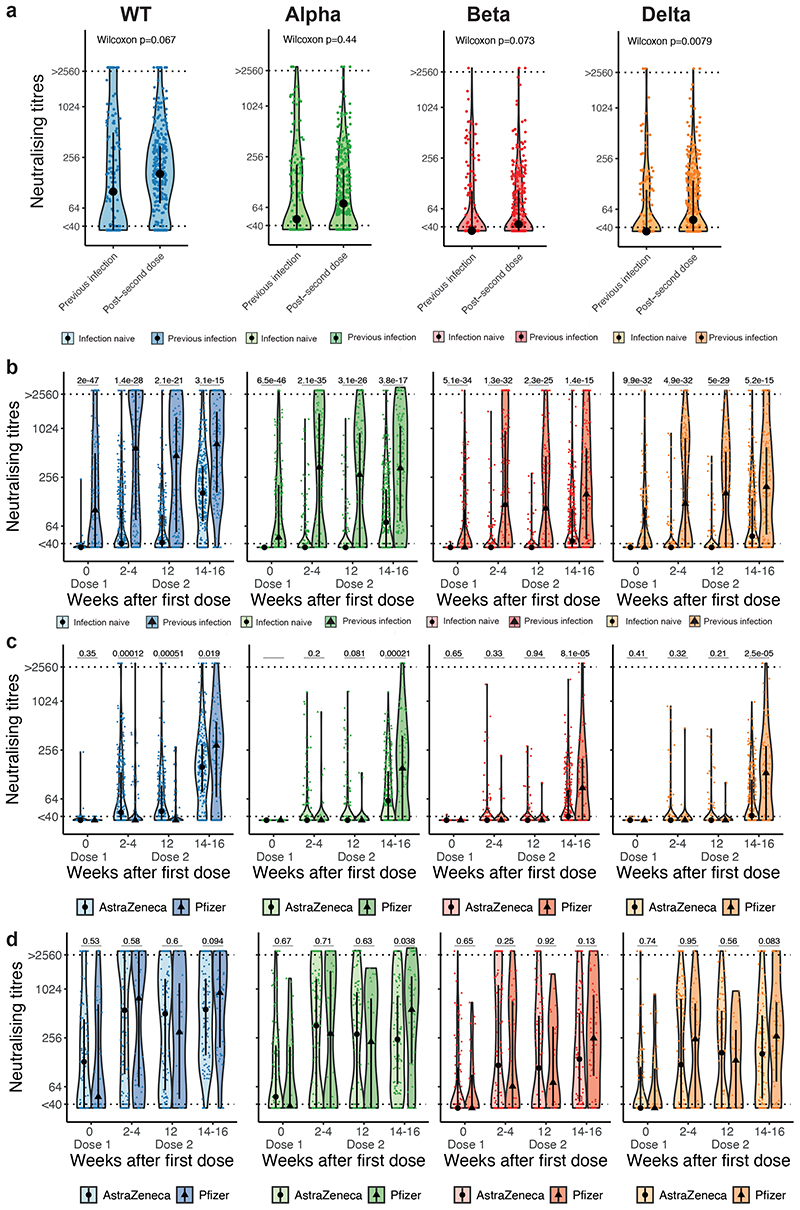
Figure 3. Neutralising response against WT SARS-CoV-2 and VOCs by prior SARS-CoV-2 infection status and type of COVID-19 vaccine.
a) Comparison of NAbT against WT SARS-CoV-2, Alpha, Beta, and Delta in patients with previous infection before vaccination vs infection naive patients post-second dose (n= 133/306 patients at BL/FU3). Significance was tested by two-sided Wilcoxon-Mann-Whitney U test, p < 0.05 was considered significant. b) Comparison of NAbT against WT SARS-CoV-2, Alpha, Beta, and Delta in infection naive (n= 318/316/253/307 patients at BL/FU1/FU2/FU3) vs patients previously infected with SARS-CoV-2 (n= 133/163/115/144 patients at BL/FU1/FU2/FU3). c) Comparison of NAbT against WT SARS-CoV-2, Alpha, Beta, and Delta in infection-naive patients receiving AZ (n= 262/246/212/229 patients at BL/FU1/FU2/FU3) vs PZ (n= 56/70/41/77 patients at BL/FU1/FU2/FU3, 1 patient with unknown vaccine type not included), and d) in patients with previous SARS-CoV-2 infection receiving AZ (n= 99/117/92/91 patients at BL/FU1/FU2/FU3) vs PZ (n=34/46/23/53) patients at BL/FU1/FU2/FU3). Dotted line at <40 denotes the lower limit of detection, dotted line at >2560 denotes the upper limit of detection. Violin plots denote density of data points. PointRange denotes the median and the 25 and 75 percentiles. Dots represent individual samples. Significance in b-d was tested by two sided Wilcoxon-Mann-Whitney U test, p < 0.05 was considered significant. AZ, AstraZeneca; NAbT, neutralising antibody titres; PZ, Pfizer; VOC, variant of concern. NA, not tested. BL, baseline; FU1, 21-56 days post first-vaccine; FU2, 14-28 days prior to second-vaccine; FU3, 1428days post second-vaccine.