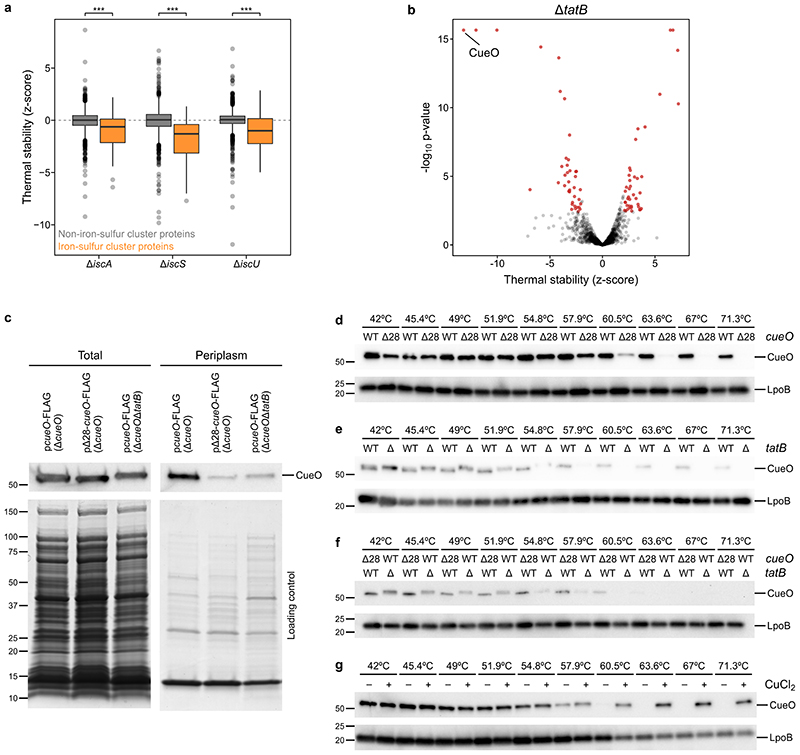
Extended Data Figure 4. Cofactor binding leads to changes in protein thermal stability.
(a) Distribution of thermal stability z-scores of all proteins in the iron-sulfur cluster biosynthesis mutants, ΔiscA, ΔiscS, and ΔiscU according to their gene ontology annotation as iron-sulfur cluster binding proteins (nΔiscA =41, nΔiscS =41, nΔiscU =40) or not (nΔiscA =1,400, nΔiscS =1,415, nΔiscU =1,314). Box plots are depicted as in Figure 2a. Significance assessed with two-sided Wilcoxon signed-rank test (p ΔiscA =3.9· 10-5, p ΔiscS =9.5·10-11, p ΔiscU =7.7·10-5). (b) Volcano plot showing proteins that significantly change in their thermal stability (highlighted in red) in ΔtatB shows that CueO is thermally destabilized. (c) Total and periplasmic protein extraction of different CueO constructs shows that deletion of Tat signal peptide (Δ28) and full-length construct in ΔtatB retain CueO protein levels, but only a small fraction makes it to the periplasm. CueO was detected using mouse monoclonal anti-FLAG antibody (F3165, Merck) and goat anti-mouse IgG-HRP (sc-2005, Santa Cruz Biotechnology) (n=1). An SDS-PAGE gel was run in parallel and stained with Coomassie to ensure that periplasmic extraction was successful (n=1). (d) Cellular thermal shift assay (CETSA) of CueO fused to FLAG peptide, either using the full length protein (WT) or a version lacking the first 28 aminoacids (Δ28; corresponding to the Tat signal peptide). Experiments performed in living cells in ΔcueO strain. CueO was detected using mouse monoclonal anti-FLAG antibody (F3165, Merck) and goat anti-mouse IgG-HRP (sc-2005, Santa Cruz Biotechnology) (n=1). As a loading control, run on the same gel, rabbit anti-LpoB antibody 3 and goat anti-rabbit IgG-HRP (sc-2004, Santa Cruz Biotechnology) were used (n=1). (e) As in panel d, but comparing the thermal stability of CueO fused to FLAG peptide, either in ΔcueO (WT) or ΔcueOΔtatB (Δ) live cells (n=1). (f) As in panel d, but comparing thermal stability of Δ28-CueO in ΔcueO strain and full length CueO in ΔcueOΔtatB (n=1). (g) CETSA of Δ28-CueO in lysate of ΔcueO strain upon addition of 4 mM CuCl2 or the same volume of vehicle (n=1). For gel source data see Supplementary Figure 2.