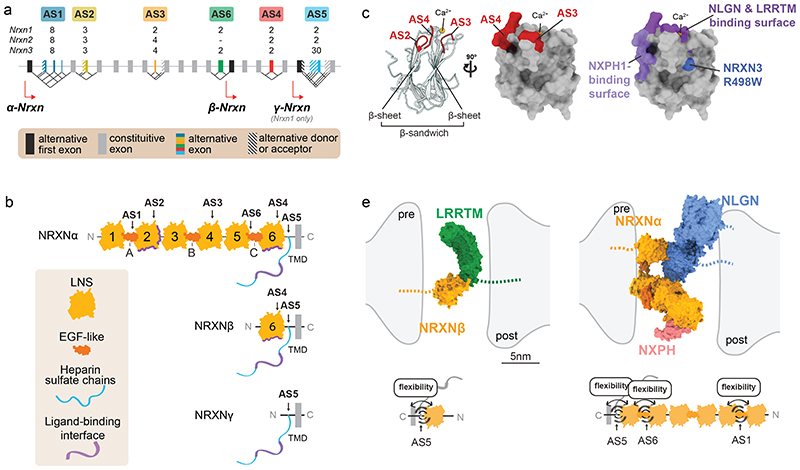
Figure 2. Molecular and structural features of Neurexin isoform diversity.
a) Schematic illustrating the alternatively spliced segments of mouse Neurexin genes. Mouse Neurexin transcript isoforms are generated from three genes (Nrxn1 = 1.1Mb, Nrxn2 = 0.1Mb, Nrxn3 = 1.8Mb; note that given the big differences in gene sizes, the exons and introns are not drawn to scale), each containing up to three alternative promoters (α, β and γ) and exhibiting extensive alternative splicing at six alternatively spliced segments (AS1-6). Individual segments can contain single alternative cassette exons (e.g. AS4, AS6) or consist of complex combinations of alternative splice donor and acceptor sites (e.g. AS1, AS2, AS3, AS5). Numbers (2,3,4,8,30) depict counts of potential splice variations generated at each segment. Alternative exons are illustrated in color, constitutive exons in grey, alternative donor or acceptor sites are striped.
b) Alternative promoters and alternative mRNA splicing result in Neurexin protein isoforms that share transmembrane domain (TMD) and cytoplasmic sequences but differ in their extracellular protein sequences. The extracellular sequences are composed of three major elements: Laminin-Neurexin-Sex hormone-binding globulin domains (LNS), epidermal growth factor-like domains (EGF), and attachment sites for heparan sulfates (HS). The largest Neurexin proteins are the NRXNα forms composed of six LNS domains (LNS 1-6), three interposed EGF domains (EGF A-C) and the HS attachment sites. Interaction surfaces for ligands are marked with purple lines. The NRXNβ forms contain a single LNS domain and HS attachment sites whereas the NRXNγ is the smallest form lacking LNS and EGF domains.
c) Mapping of alternatively spliced segments (AS2, AS3, AS4), ligand-binding domains, and sequence variants on a prototypical LNS domain: (left) ribbon diagram and positions of alternatively spliced segments, (middle) view of a 90-degree rotation and surface representation LNS domain with mapped alternatively spliced segments, (right) surface representation as in middle panel highlighting the position of ligand-binding domains and the naturally occurring R498W variant in NRXN3α which has been linked to behavioral alterations in mice 129 .
d) Illustration of approximate sizes and hypothetical conformation of adhesion molecule complexes in the synaptic cleft: β-Neurexin (orange, left panel) with LRRTM2 (green), and α-Neurexin (orange, right panel) with Neurexophilin (NXPH 1, pink) and Neuroligin (NLGN, blue). Structural models of the extracellular domains were drawn with ChimeraX 1.0 from the following Protein Data Bank IDs: 3POY 48 , 3B3Q 53 , 6PNP 50 , 5Z8Y 139 . The position of stalk, transmembrane and cytoplasmic sequences is indicated as dashed lines. Diagrams at the bottom display positions within these structures where alternative splicing at AS1, AS6 and AS5 in NRXN β (left) and NRXN α (right) modifies the flexibility of the extracellular domains in the synaptic cleft