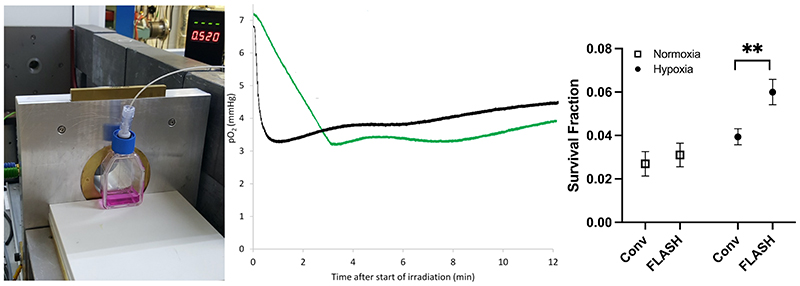
Figure 3.
The setup used (left), the measurement results (middle) of oxygen consumption (measured in media with an Oxylite, Oxford Optronics, Oxford, UK), and clonogenic cell survival (right), following delivery of 20 Gy with ultra-high (2000 Gy/s, black trace) and conventional dose rates (0.1 Gy/s, green trace) to a T12.5 cell flask with a monolayer of H454 murine glioblastoma cells and 5 ml media, from a horizontal 6 MeV electron beam. The beam-on time for FLASH is just 0.01 s. So, for an ideal measurement probe the black trace should drop in this short time but because of averaging of the signal, there is a delay of several seconds. For the same reason, small shoulders are visible for the green trace where the irradiation starts and stops (at around 3 min and 10 s). Similar to data from Cao et al. (Cao et al. 2021), the oxygen consumption is clearly greater for the conventional dose rate, i.e. the p02 value reduction was greater for the green trace (4.0 mmHg, i.e. 0.20 mmHg/Gy) than the black trace (3.5 mmHg, i.e. 0.17-0.18 mmHg/Gy), though the timescale of reduction is very different. Some diffusion of oxygen into the media from the gas in the flask (where the oxygen consumption is far less prominent than in the media) is evident after the end of irradiation. Clonogenic cell survival data show a small non-significant sparing for FLASH irradiation vs. conventional dose rate (CONV) irradiation when cells are prepared in normoxia, which expands and becomes significant (**, p<0.01) when cells are prepared in hypoxia (6 mmHg, following 1-2h in a hypoxia chamber prior to irradiation).