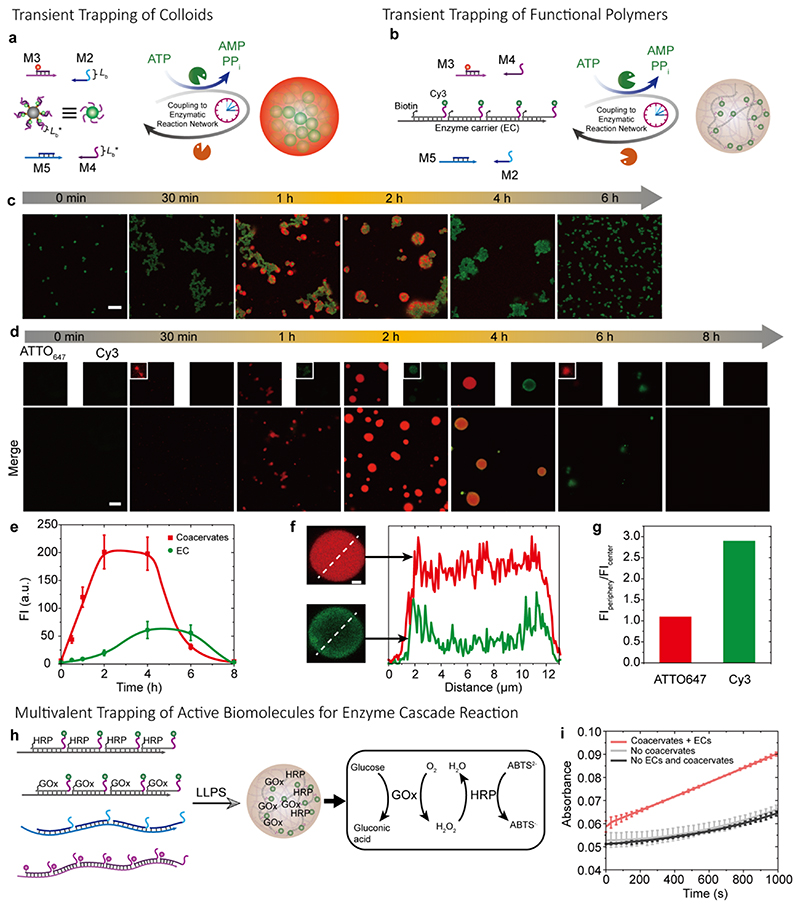
Figure 5. Multivalency-driven superstructures and catalytically active coacervates.
(a) Schematic illustration of ATP-driven transient coacervates for temporal trapping of micron-sized colloids. (b) Schematic illustration for ATP-driven transient trapping of functional DNA polymers in the DNA coacervates via multivalency. (c) Time-dependent CLSM of the transient colloid assembly and transient coacervates. CLSM measurements were conducted consecutively with the same microscopy settings. Scale bar = 10 μm. (d) Time-dependent CLSM measurements of the transient coacervates with transient trapping and releasing functional DNA polymers via transient multivalency. CLSM measurements were conducted consecutively with the same microscopy settings. Scale bar = 10 μm. (e) Time-dependent fluorescence intensities for the transient coacervates and entrapped fluorescent DNA polymers. Error bars are standard deviations from three random fields of view of duplicate experiments. Lines are guides to the eye. (f) Fluorescence intensity profiles for coacervates and entrapped functional DNA polymers at 4 h. Scale bar = 2 μm. (g) Quantified fluorescence intensity (FI) ratios of the FIperiphery to the FIcenter of the coacervates. (h) Schematic illustration of GOx and HRP encapsulation in the coacervates. (i) Catalytic activity of the multienzyme complex inside the coacervates in comparison to free enzymes and enzymes on enzyme carriers (EC) but without coacervates. Reactions monitored by absorbance at 414 nm. Error bars are standard deviations of duplicate measurements. Conditions for (a): 37°C, 0.01 mM M5, 0.012 mM M2, 0.01 mM M3, 0.012 mM M4, ca. 4.68-6.68×107 beads/mL, 0.92 WU/μL T4 DNA ligase, 1.0 U/μL BsaI, and 0.06 mM ATP, under orbital shaking at 80 rpm. Conditions for (b): 37°C, 0.01 mM M5, 0.012 mM M2, 0.01 mM M3, 0.012 mM M4, 0.92 WU/μL T4 DNA ligase, 1.0 U/μL BsaI, ca. inert functional polymers of 2 nM repeating units, and 0.06 mM ATP, under orbital shaking at 80 rpm. Conditions for (h): 37°C, 0.5 nM GOx on enzyme carriers, 0.5 nM HRP on enzyme carriers, 2 mM ABTS2-, SfNAP1 and SfNAP2 of 0.008 mM repeating units, under orbital shaking at 97 rpm. After coacervating for 2 h, 1 mM glucose was added, and absorbance at 414 nm was measured.