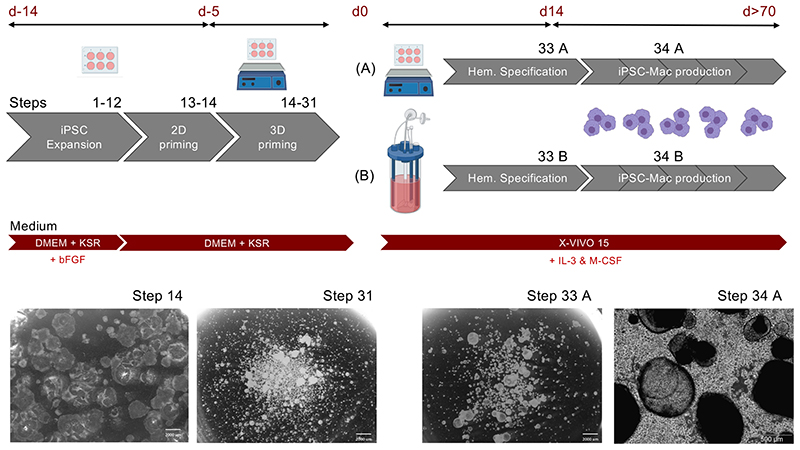
Figure 1. Schematic representation of the differentiation platform.
Human iPSC are expanded in 2D adherent cultures using DMEM+KSR+bFGF (“Full iPSC medium”), followed by a 2D priming phase in DMEM+KSR (“minimal iPSC medium), both performed on murine embryonic fibroblasts (MEFs) without intermediate passaging. Subsequently, fragmented colonies are transferred to 3D suspension culture in a 6-well plate on an orbital shaker for 3D priming in minimal iPSC medium. After 5 days, the largest primed aggregates are selected by defined sedimentation and transferred (A) to a new 6-well suspension culture plate and maintained on the orbital shaker in a culture volume of 3 ml/well or (B) to a stirred tank bioreactor in a culture volume of 120 ml. By cultivation of primed aggregates in X-VIVO15 medium supplemented with IL-3 and M-CSF for 14 days (Hematopoietic specification) myeloid cell forming complexes (MCFCs) develop. From day 14 onwards, iPSC-derived macrophages can be harvested in parallel to the medium change, while MCFCs remain in the process. Abbreviations: iPSC: Induced pluripotent stem cell, DMEM: Dulbecco’s Modified Eagle’s Medium, KSR: Knock our serum replacement, bFGF: basic fibroblast growth factor, IL-3: Interleukin 3, M-CSF: Macrophage colony stimulating factor. Scale bars: 2000 μm/ magnification 6.1x and 500 μm, magnification 40x.