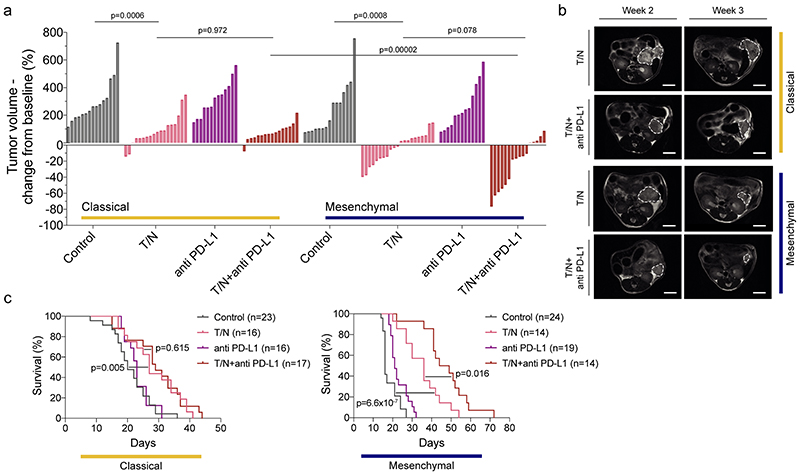
Figure 6. The trametinib/nintedanib combination sensitizes mesenchymal PDAC towards anti PD-L1 immune checkpoint blockade.
a, Waterfall plot showing tumor response of classical and mesenchymal PDAC to T/N+anti PD-L1 vs vehicle control, T/N, and baseline anti PD-L1 therapy after one week of treatment (values represent fold-change compared to baseline before treatment based on MRI-volumetric measurements, y axis). P values calculated with two-tailed unpaired t test.
b, Representative MRI of vehicle and T/N+anti PD-L1 treated mice before (week 2) and after 1 week treatment (week 3). Scale bar, 5 mm.
c, Kaplan-Meier survival curves of classical and mesenchymal orthotopically transplanted models of the indicated treatment arms. The number of mice is indicated in the corresponding panels. P value was calculated with log-rank (Mantel-Cox) test.
T: trametinib, N: nintedanib, T/N: trametinib+nintedanib, T/N+anti PD-L1: trametinib+nintedanib+anti PD-L1 antibody.
Note: The classical and mesenchymal cohorts, control and T/N, in panel (a) and (c) are the same shown in figure 3, panels (a), (b) and (d, left and middle panel).