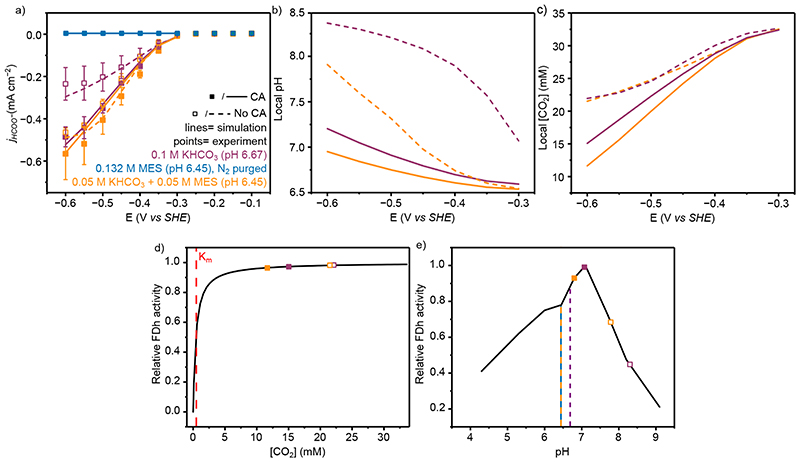
Figure 4. The electrochemical performance and simulated local environment of FDh with and without CA co-immobilisation.
(a) Experimental and simulated partial current densities for HCOO− quantified by ion chromatography from controlled potential electrolysis of 50 pmol FDh immobilised on a mesoporous ITO electrode. Points represent averages of at least three independent stepped-chronoamperometry experiments, where filled circles used co-immobilised CA (40 pmol) on the electrode surface and unfilled no CA. Error bars represent the standard deviation (b) Average pH and (c) CO2 concentration within the mesoporous electrode from the FEM at steady state. For a-c lines represent simulated results from the FEM, with solid lines are with CA and dashed lines without. (d) Effect of CO2 concentration on CO2R activity. The line represents the enzyme Michaelis-Menten kinetics determined from solution assay (KM = 0.420 mM) 17 and the points are the concentrations used in this work. e) Effect of pH on activity. The line represents the enzyme pH activity determined from solution assay 17 and the points are the average values from FEM within the mesoporous electrode for the system used. Dashed vertical lines are the bulk solution pH. Solution conditions; Purple: CO2 purged 0.1 M KHCO3 and 0.05 M KCl (pH 6.67); Orange: CO2 purged 0.05 M KHCO3, 0.05 M MES and 0.05 M KCl (pH 6.45); Blue: N2 purged 0.132 M MES + 0.05 M KHCO3 (pH 6.45). All experiments conducted at 20°C.