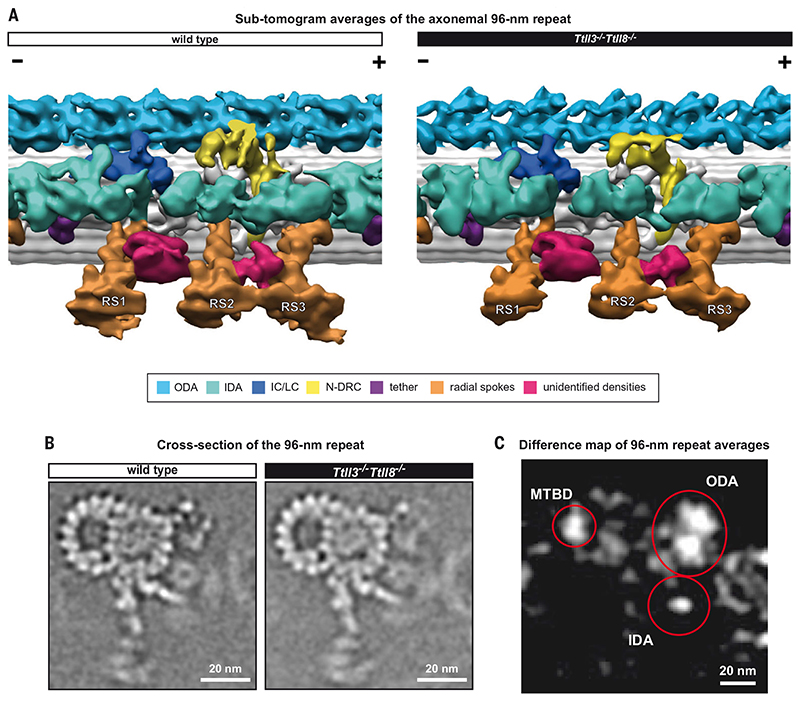
Fig. 5. The assembly of the axonemal 96-nm repeat is not affected in Ttll3 −/− Ttll8 −/− sperm.
(A) 3D-isosurface rendering of the 96-nm repeats from active wild-type and Ttll3 −/− Ttll8 −/− sperm flagella after subtomogram averaging. All known components of the axonemal 96-nm repeat were identified in both wild type and Ttll3 −/− Ttll8 −/−, indicating that absence of glycylation did not affect their assembly. We further identified densities of structures that are not found in axonemes from other species (crimson-colored). A barrel-shaped structure between radial spokes 1 and 2 (RS1 and RS2) and a density that links the neck of radial spokes 2 and 3 (RS2 and RS3). ODA, outer dynein arm; IDA, inner dynein arm; IC/LC, dynein intermediate chain/light chain; N-DRC, nexin-dynein regulatory complex. (B) Slice through subtomogram averaged wild-type and Ttll3 −/− Ttll8 −/− 96-nm repeats. No evident modifications to the macromolecular assembly of the axoneme were found upon depletion of tubulin glycylation. (C) Difference map of wild-type and Ttll3 −/− Ttll8 −/− 96-nm repeat averages. Areas of the 96-nm repeat that present meaningful structural differences between the two averages are circled in red. These include ODAs, IDAs, and part of the external wall of the B-tubule. MTBD, microtubule-binding domain.