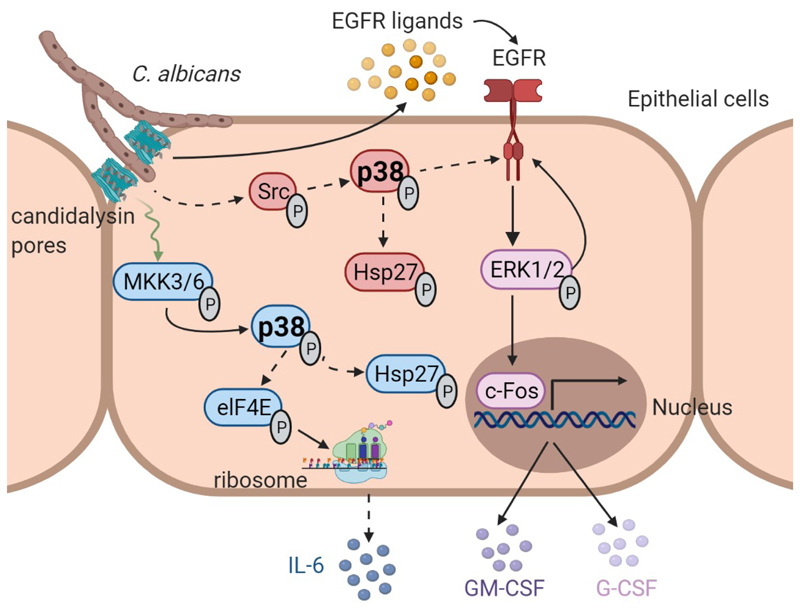
Fig. 8. Graphical summary.
Candidalysin is secreted into an invasion pocket formed by C. albicans hyphae. Candidalysin intercalates into the host cell membrane and stimulates the release of EGFR ligands, which induces ERK1/2 activation downstream of EGFR, resulting in c-Fos activation and the release of the neutrophil-activating chemokines GM-CSF and G-CSF. Through a parallel pathway, candidalysin also activates p38, resulting in IL-6 release and Hsp27 phosphorylation. p38 activation is not triggered by EGFR but by two selective and independent pathways that differentially control p38 signaling outputs. MKK3/6 promote IL-6 release, whereas Src triggers p38-induced EGFR phosphorylation independent of ligand binding. Both Src and MKK3/6 promote p38-dependent Hap27 phosphorylation. Created with BioRender.com