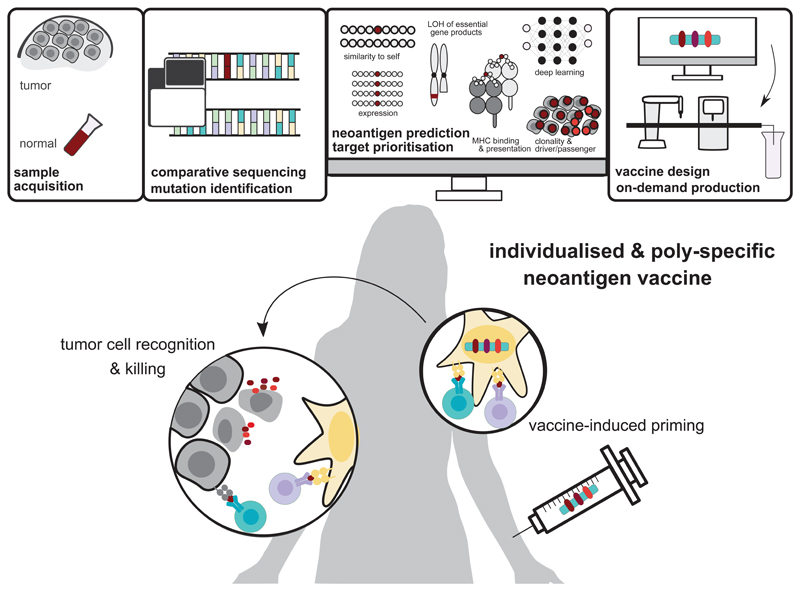
Figure 1. Engineering individualised neoantigen vaccines.
Next-generation sequencing of a patient’s healthy tissue (e.g., PBMC, peripheral blood mononuclear cells) and tumour biopsies is performed. The sequencing data from tumour and normal DNA is compared to identify tumour-specific mutations. Mutations are prioritised as vaccine candidates based on their likelihood to elicit a T-cell response by computational methods such as MHC binding prediction, quantification of mutated transcript expression, clonality of the mutation and other features. Using the vaccine platform of choice (e.g. mRNA, long peptides) an individualised and poly-specific neoantigen vaccine is manufactured on-demand under GMP conditions. Neoantigen vaccination aims at restoring the cancer immunity cycle by inducing de novo T-cell responses that induce tumour killing and by supporting the shift from ignorance toward anti-tumour immunity. LOH: loss of heterozygosity, GMP: good manufacturing practice