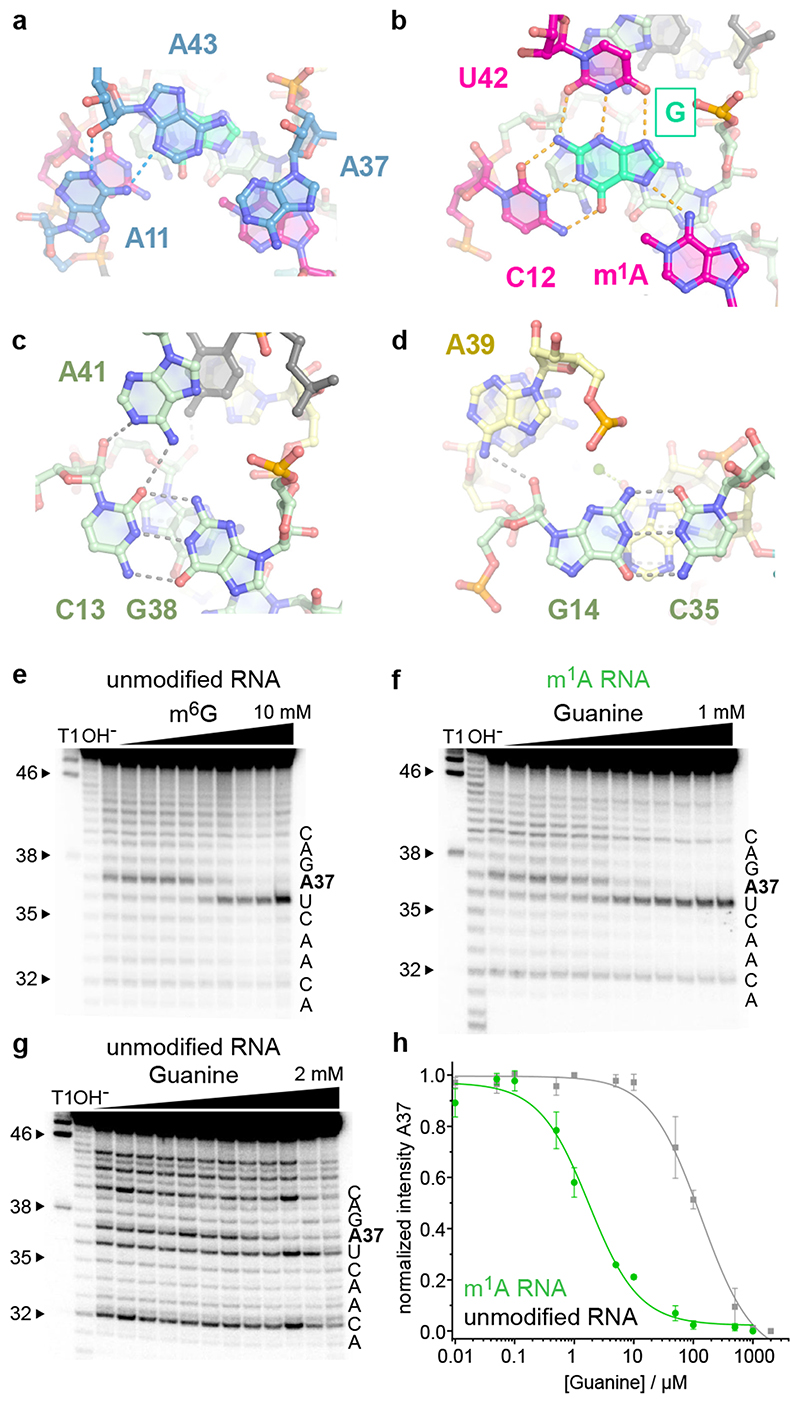
Fig.2. The catalytic core domain of MTR1.
(a) – (d) Stick representations of the four layers of the core domain from top to bottom: (a) adenosine triple A11, A37, A43. (b) active site with m1A and G interacting by base pairing with C12 and U42. (c) C13:G38 base pair with A-minor interaction of A41. (d) G14:C35 base pair interacting with A39. (e-g) Representative excerpts of in-line probing gels of MTR1 (pH 8.0, 20 °C, 36 h): (e) hybridized to unmodified R1 in the absence and presence of m6G (0.5 μM–10 mM). (f) hybridized to methylated m1A-RNA, or (g) unmodified R1 in the absence and presence of guanine (0.01–1000 μM in (f), 2000 μM in (g). (h) Normalized in-line probing band intensities for A37 in the m1A-containing RNA complex (f, green) compared to unmodified RNA (g, grey). The lines represent a fit to a one-site binding model. Error bars denote ± s.d. of the mean for n = 3 (grey) or 4 (green) independent replicates. See Extended Data Fig 3 for full gel images and additional analyses for U36 and G38.