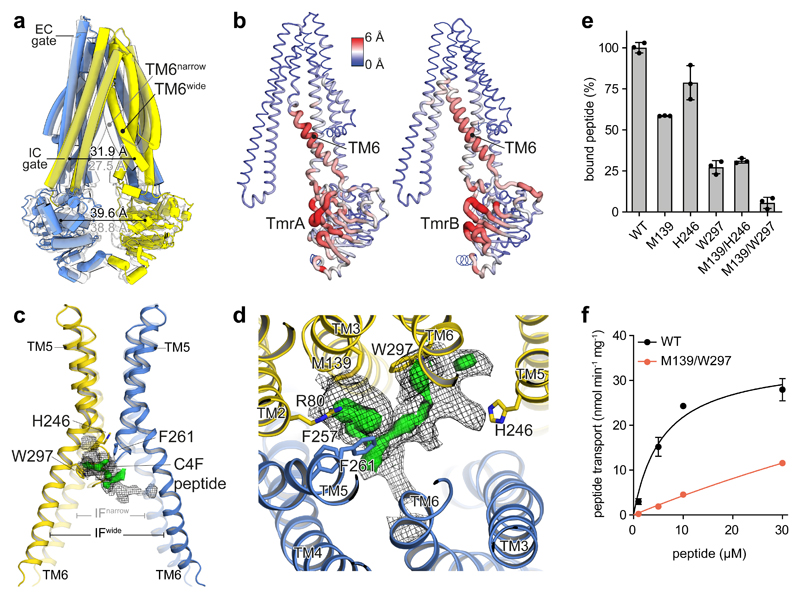
Figure 2. Conformational plasticity in IF conformations translates to substrate binding.
a, Superposition of the IFnarrow (grey) and IFwide conformation illustrating the displacement of TM6. In IFwide, the intracellular (IC) gate opens by 4.4 Å, while the inter-NBD distance (center of mass) changes only marginally. TmrA is blue, TmrB yellow. b, The TM6 gatekeeper helix and the NBD interface show highest conformational variability in the IF state. Red color and wide tubes indicate large Cα distances between IFnarrow and IFwide in a backbone superposition, excluding the N-terminal elbow and C-terminal helices. c, Superposition of IFwide and IFnarrow (transparent) and focussed representation of the substrate-binding region of TmrAB highlights the widening of the binding cavity upon displacement of TM6. The helix acts as a gatekeeper for substrate uptake, blocking binding of bulky substrates in the narrow conformation. The calculated difference map (Fobs – Fcalc, green) is overlaid with the experimental density (mesh) at a lower threshold to illustrate the extent of the putative peptide density. d, Focussed top view with cryo-EM density (mesh) and positive difference density (green surface) in the binding site of IFwide (TmrABC4F), as also identified in TmrABturnover, but not in TmrABapo. Nearby residues are depicted as sticks colored by heteroatoms. e, Alanine substitutions in TmrB reduce substrate binding, as analyzed by fluorescence polarization of 50 nM C4F peptide and 2 μM TmrAB. f, Peptide transport kinetics of TmrABWT (black) and double alanine mutant (red) show an additive effect. Binding and transport data represent mean ± SD from three experiments.