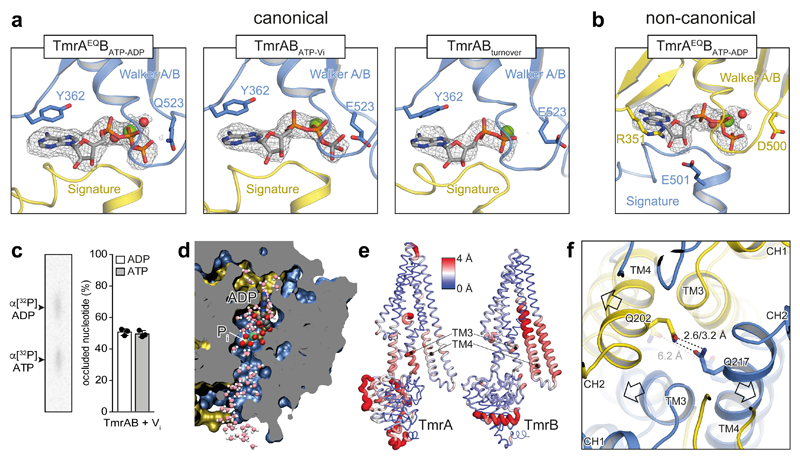
Figure 3. Nucleotide states in the sandwiched NBD dimer and opening of the intracellular gate.
a, Different nucleotide states were observed at the canonical nucleotide-binding site in the conformations with dimerized NBDs from TmrAEQBATP-ADP, TmrABATP-Vi, and TmrABturnover (highest resolution map presented). In all cases, the nucleotide-binding pocket is aligned by the conserved Walker A/B motives and the signature motif. Mg2+ and water molecules are shown as green and red spheres, respectively. TmrA is blue, TmrB yellow. b, The non-canonical site is occupied by ATP in all examples, as hydrolysis cannot proceed. OFopen from TmrAEQBATP-ADP is shown as an example. In the non-canonical site, the sugar moiety of the bound nucleotide interacts with R351TmrB and E501TmrA, while a tyrosine residue (Y362TmrA) makes π-π stacking contacts with the purine base in the canonical site. c, The stoichiometric occlusion of ADP and ATP was validated by thin-layer chromatography upon vanadate-trapping of TmrABWT (mean ± SD from three experiments). d, MD snapshot showing two possible exit pathways for inorganic phosphate (Pi) after hydrolysis in a cut through the NBD dimer. Pi can escape through hydrated channels directed towards the C-terminal helices (bottom) and the coupling helix (top). The OFoccluded conformation of TmrABATP-Vi was simulated with bound ADP and Pi (HPO42–). e, The NBD dimer, TM3, and TM4 show the largest differences between OFoccluded and URasym* conformations. Red color and wide tubes indicate large Cα distances. The distances were averaged over two OFoccluded (from TmrAEQBATP-ADP and TmrABATP-Vi) and the URasym* conformation in a backbone superposition excluding the N-terminal elbow and C-terminal helices. The TM3 and TM4 motions from OF to URasym* progressively separate the intracellular gate. f, After Pi release, the intracellular gate lined by TM4 and TM3 of each subunit is unlocked, forming the asymmetric turnover state URasym*. OFoccluded is transparent.