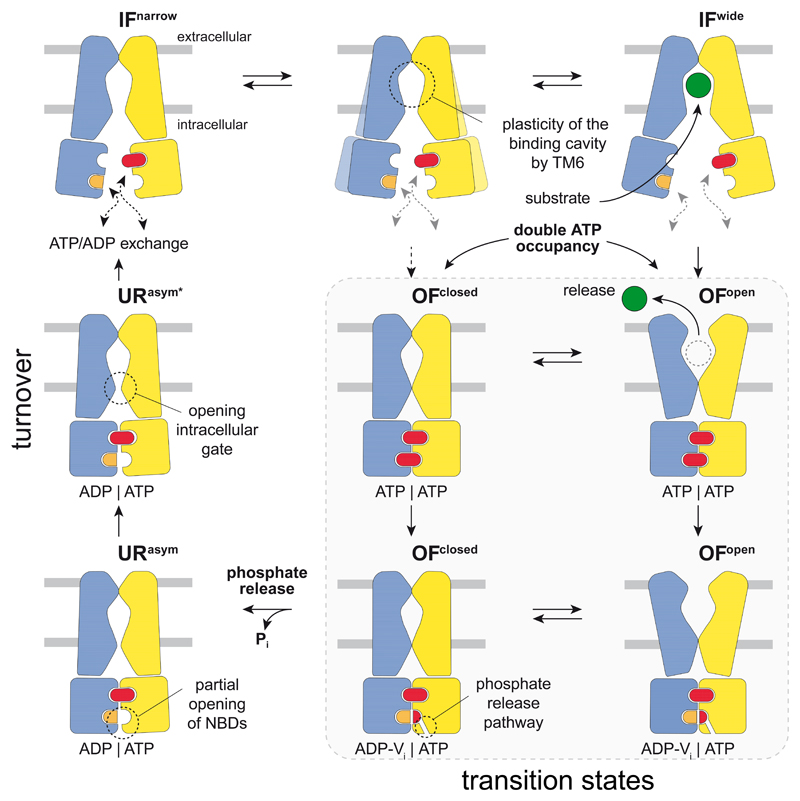
Figure 4. Translocation cycle of the heterodimeric ABC exporter TmrAB.
TmrAB fluctuates between IFnarrow and IFwide with TM6 acting as gatekeeper, controlling access and volume of the substrate-binding cavity. Therefore, bulky substrates can only bind to the IFwide conformation. Independent of substrate binding and IF conformation, ATP binding to both NBDs induces NBD dimerization, which closes the intracellular gate and allows the extracellular gate t0 open. After substrate release, TmrAB can isomerize between OFopen and OFoccluded conforoations in the ATP-bound state until Pi is discharged from the canonical site. Pi release via a phosphate channel results in an asymmetric URasym conformation and triggers partial opening of the NBDs and the intracellular gate, which advances the exporter towards the IF conformation. Separation of the NBD dimer by dissociation of the ATP-bound non-canonical site represents the rate-limiting step during the cycle. Conceivably, ATP binding can also lead directly to an OFoccluded conformation. In this case bulky substrates cannot be transported, indicating either a futile cycle or one designated for small, amphipathic molecules, that do not require opening of the extracellular gate for transport. TmrA and TmrB are shown in blue and yellow, respectively.