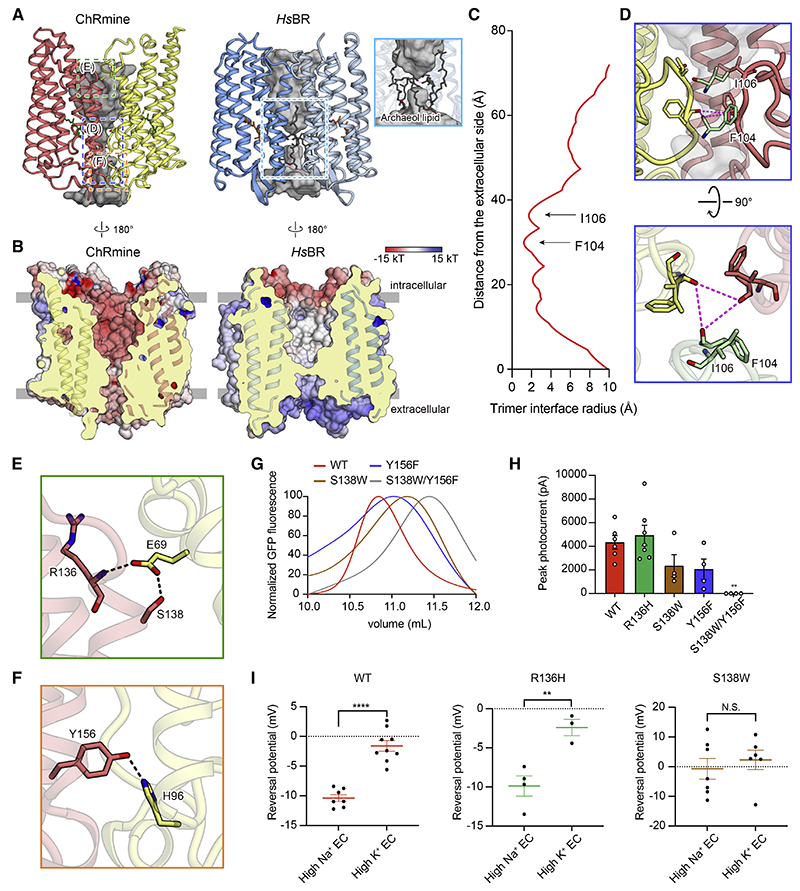
Figure 4. The functional importance of trimeric assembly.
(A and B) The opening within the trimer interface in ChRmine (left) and HsBR (right). Opening: gray (A). Electrostatic potential surface (B).
(C) Trimer opening radii of the ChRmine.
(D) Magnified views of the blue-boxed region from (A), the constriction formed by ECL1, from two angles.
(E and F) Magnified views of the green- and orange-boxed regions in (A), the H-bond interactions between protomers at the intracellular (E) and extracellular
(F) side.
(G) FSEC traces of ChRmine WT, S138W, Y156F, and S138W/Y156F mutants.
(H) Photocurrent amplitudes for the mutants shown in (E) and (F), as well as R136H (negative control) (n = 4−7).
(I) Reversal potentials of ChRmine WT, R136H, and S138W mutants (n = 3–9). All data mean ± SEM; sample size n denotes the number of cells. One-way ANOVA with Dunnett’s test (I) and unpaired t test (H), **p < 0.01 and ****p < 0.0001. N.S., not significant.